A Morphological Study of the Multi-Posterior Superior Alveolar Canals of Maxilla in the Japanese Macaque by Cone-Beam Computed Tomography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Para Nasal Sinusitis : a Problem for the General Practitioner
University of Nebraska Medical Center DigitalCommons@UNMC MD Theses Special Collections 5-1-1933 Para nasal sinusitis : a problem for the general practitioner Robert James Ralston University of Nebraska Medical Center This manuscript is historical in nature and may not reflect current medical research and practice. Search PubMed for current research. Follow this and additional works at: https://digitalcommons.unmc.edu/mdtheses Recommended Citation Ralston, Robert James, "Para nasal sinusitis : a problem for the general practitioner" (1933). MD Theses. 283. https://digitalcommons.unmc.edu/mdtheses/283 This Thesis is brought to you for free and open access by the Special Collections at DigitalCommons@UNMC. It has been accepted for inclusion in MD Theses by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. PARA-NASAL SINUSITIS A PROBLEM FOR THE GENERAL PRACTITIONER by Rooert James Ralston, B. Sc. Presented to the Faculty of The College of Medicine in the University of Nebraska in Partial Fulfillment of the Requirements - for the Degree of Doctor of Medicine 1933 Omaha, Nebraska TABLE O.F' CONTENTS Pages I Introductory and Historioal 1 - 2 II Anatomioal Discussion of Nose and Accessory Nasal Sinuses 3 - 16 III Etiology and Inoidence of Paranasal Sinus Infection 17 - 20 IV Pathology of Paranasal Sinusitis 21 - 23 V Sinus Infection in Relation to Systemic Disease 24 - 28 VI Symptoms and Diagnosis of Paranasal Sinusitis 29 - 38 VII Treatment of Paranasal Sinusitis 39 - 45 VIII Case Histories 46 - 55 IX Conclusion 56 X Bibliography 1 INTRODUOTORY AND HISTORICAL The paranasal, or accessory sinuses of the nose, are as sociated with the general health of the individual just as closely as are the tonsils, the teeth, the gall bladder, or any other of the commonly accepted foci of infection in the human body. -

Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Chapter 2 Implants and Oral Anatomy
Chapter 2 Implants and oral anatomy Associate Professor of Maxillofacial Anatomy Section, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University Tatsuo Terashima In recent years, the development of new materials and improvements in the operative methods used for implants have led to remarkable progress in the field of dental surgery. These methods have been applied widely in clinical practice. The development of computerized medical imaging technologies such as X-ray computed tomography have allowed detailed 3D-analysis of medical conditions, resulting in a dramatic improvement in the success rates of operative intervention. For treatment with a dental implant to be successful, it is however critical to have full knowledge and understanding of the fundamental anatomical structures of the oral and maxillofacial regions. In addition, it is necessary to understand variations in the topographic and anatomical structures among individuals, with age, and with pathological conditions. This chapter will discuss the basic structure of the oral cavity in relation to implant treatment. I. Osteology of the oral area The oral cavity is composed of the maxilla that is in contact with the cranial bone, palatine bone, the mobile mandible, and the hyoid bone. The maxilla and the palatine bones articulate with the cranial bone. The mandible articulates with the temporal bone through the temporomandibular joint (TMJ). The hyoid bone is suspended from the cranium and the mandible by the suprahyoid and infrahyoid muscles. The formation of the basis of the oral cavity by these bones and the associated muscles makes it possible for the oral cavity to perform its various functions. -
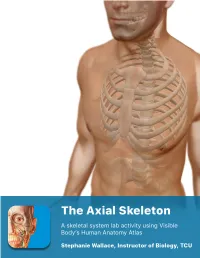
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -

Atlas of the Facial Nerve and Related Structures
Rhoton Yoshioka Atlas of the Facial Nerve Unique Atlas Opens Window and Related Structures Into Facial Nerve Anatomy… Atlas of the Facial Nerve and Related Structures and Related Nerve Facial of the Atlas “His meticulous methods of anatomical dissection and microsurgical techniques helped transform the primitive specialty of neurosurgery into the magnificent surgical discipline that it is today.”— Nobutaka Yoshioka American Association of Neurological Surgeons. Albert L. Rhoton, Jr. Nobutaka Yoshioka, MD, PhD and Albert L. Rhoton, Jr., MD have created an anatomical atlas of astounding precision. An unparalleled teaching tool, this atlas opens a unique window into the anatomical intricacies of complex facial nerves and related structures. An internationally renowned author, educator, brain anatomist, and neurosurgeon, Dr. Rhoton is regarded by colleagues as one of the fathers of modern microscopic neurosurgery. Dr. Yoshioka, an esteemed craniofacial reconstructive surgeon in Japan, mastered this precise dissection technique while undertaking a fellowship at Dr. Rhoton’s microanatomy lab, writing in the preface that within such precision images lies potential for surgical innovation. Special Features • Exquisite color photographs, prepared from carefully dissected latex injected cadavers, reveal anatomy layer by layer with remarkable detail and clarity • An added highlight, 3-D versions of these extraordinary images, are available online in the Thieme MediaCenter • Major sections include intracranial region and skull, upper facial and midfacial region, and lower facial and posterolateral neck region Organized by region, each layered dissection elucidates specific nerves and structures with pinpoint accuracy, providing the clinician with in-depth anatomical insights. Precise clinical explanations accompany each photograph. In tandem, the images and text provide an excellent foundation for understanding the nerves and structures impacted by neurosurgical-related pathologies as well as other conditions and injuries. -

Columna Vertebralis Thorax
WSKAZÓWKI DO ĆWICZEŃ DLA STUDENTÓW WYDZIAŁU LEKARSKIEGO Zakład Anatomii Prawidłowej i Klinicznej CB AM w Warszawie B.Ciszek Wymienione poniżej miana anatomiczne wskazują struktury anatomiczne, które należy umieć rozpoznać i omówić. Obowiązujące są miana łacińskie i angielskie. OSTEOLOGIA & ARTHROLOGIA V OS TEMPORALE TEMPORAL BONE Pars petrosa Petromastoid part Margo occipitalis Occipital border Processus mastoideus Mastoid process Incisura mastoidea Mastoid notch Sulcus sinius sigmoidei Sulcus for sigmoid sinus Sulcus arteriae occipitalis Occipital groove Foramen mastoideum Mastoid foramen Canalis facialis Facial nerve canal Geniculum canalis facialis Geniculum Canaliculus chordae tympani Canaliculus for the chorda tympani Apex partis petrosae Apex Canalis caroticus Carotid canal Canaliculi caroticotympanici Caroticotympanic canaliculus Canalis musculotubarius Semicanalis m.tensoris tympani Canal for the tensor tympani Semicanalis tubae auditivae Osseous part of the pharyngotympanic Septum canalis musculotubarii Septum /tube Facies ant.partis petrosae Anterior surface Tegmen tympani Tegmen tympani Eminentia arcuata Arcuate eminence Hiatus canalis nervi Hiatus for the greater petrosi maioris petrosal nerve Sulcus n.ptr.maioris Groove for the greater ptr.n. Hiatus canalis nervi Hiatus for the minor petrosal nerve petrosi minoris Sulcus n.ptr.minoris Groove for the minor ptr.n. Impresio trigeminalis Trigeminal impression Margo sup.partis petrose Superior border Sulcus sinus petrosi sup. Sulcus for sup. petrosal sinus Facies post.partis petrosae -

Splanchnocranium
splanchnocranium - Consists of part of skull that is derived from branchial arches - The facial bones are the bones of the anterior and lower human skull Bones Ethmoid bone Inferior nasal concha Lacrimal bone Maxilla Nasal bone Palatine bone Vomer Zygomatic bone Mandible Ethmoid bone The ethmoid is a single bone, which makes a significant contribution to the middle third of the face. It is located between the lateral wall of the nose and the medial wall of the orbit and forms parts of the nasal septum, roof and lateral wall of the nose, and a considerable part of the medial wall of the orbital cavity. In addition, the ethmoid makes a small contribution to the floor of the anterior cranial fossa. The ethmoid bone can be divided into four parts, the perpendicular plate, the cribriform plate and two ethmoidal labyrinths. Important landmarks include: • Perpendicular plate • Cribriform plate • Crista galli. • Ala. • Ethmoid labyrinths • Medial (nasal) surface. • Orbital plate. • Superior nasal concha. • Middle nasal concha. • Anterior ethmoidal air cells. • Middle ethmoidal air cells. • Posterior ethmoidal air cells. Attachments The falx cerebri (slide) attaches to the posterior border of the crista galli. lamina cribrosa 1 crista galli 2 lamina perpendicularis 3 labyrinthi ethmoidales 4 cellulae ethmoidales anteriores et posteriores 5 lamina orbitalis 6 concha nasalis media 7 processus uncinatus 8 Inferior nasal concha Each inferior nasal concha consists of a curved plate of bone attached to the lateral wall of the nasal cavity. Each consists of inferior and superior borders, medial and lateral surfaces, and anterior and posterior ends. The superior border serves to attach the bone to the lateral wall of the nose, articulating with four different bones. -

Prevalence of Mandibular Retromolar Canal on Cone Beam Computed Tomography Scans
Original Article Prevalence of Mandibular Retromolar Canal on Cone Beam Computed Tomography Scans K. Amini 1, R. Ghafari 2, SH. Kazemi 3 , F. Bagheri 4. 1 Assistant Professor, Department of Endodontics, School of Dentistry, Islamic Azad University Isfahan (Khorasgan) Branch, Isfahan, Iran 2 Postgraduate student, Department of Oral Radiology, School of Dentistry, Islamic Azad University Isfahan (Khorasgan) Branch, Isfahan, Iran 3 Postgraduate student, Department of Endodontics, School of Dentistry, Islamic Azad University Isfahan (Khorasgan) Branch, Isfahan, Iran 4 Dentist, Private Office, Qom, Iran Abstract Background and Aim: The retromolar canal is an anatomical structure in the mandible and a type of bifid inferior alveolar canal. The retromolar canal may provide accessory innervation to the mandibular molars or contain an aberrant buccal nerve; thus, this canal is of clinical significance. The aim of this study was to evaluate the prevalence of retromolar canal on cone-beam computed tomography (CBCT) scans. Materials and Methods: In this descriptive-analytical study, 151 inferior alveolar canals in 102 patients (102 unilateral and 49 bilateral) with third molar teeth requiring CBCT scans were evaluated. The scans were evaluated for presence of the retromolar canal and linear measurements (distance to second molar, height and width) were made. The data were analyzed paired t-test and chi square test. Results: The prevalence of retromolar canal was 7.3% (n=11). With regard to linear Corresponding author: measurements, the mean distance from the retromolar canal to the second molar was SH. Kazemi, Postgraduate 12.76± 4.3mm. The mean height of the canal was 6.66 ±2.18mm, and the mean width student, Department of was 1.7± 0.6mm. -

Cephalometric Superimpositions
Original Article Cephalometric Superimpositions A Comparison of Anatomical and Metallic Implant Methods Yan Gu a; James A. McNamara Jrb ABSTRACT Objective: To test the hypothesis that there is no difference between the information produced by superimposition of serial lateral headfilms on anatomical structures and that produced by su- perimposition on metallic implants according to the protocols of Bjo¨rk. Materials and Methods: Serial cephalograms of 10 untreated subjects who had tantalum implants placed in the maxilla and mandible during childhood were analyzed. Headfilms taken at six con- secutive stages of cervical vertebral maturation (CS1-CS6) for six female and four male subjects were used. Tracings were superimposed according to the methods of superimpositions advocated by the American Board of Orthodontics (ABO). In addition, superimpositions along the inferior border of the mandible were performed. Finally, superimpositions of serial tracings on stable intraosseous implants were made to determine the actual growth and remodeling patterns of the maxilla and mandible. Results: The ABO maxillary superimposition method underestimates the vertical displacement and overestimates the forward movement of maxillary landmarks. Superimposing on the internal cortical outline of the symphysis and the inferior alveolar nerve canals generally approximates the mandibular superimposition on implants, although the lower anterior border of the symphysis may be a preferable area of superimposition. Superimposition on the lower border of the mandible does not reflect accurately the actual pattern of growth and remodeling of the mandible. Conclusions: When analyzing serial headfilms, erroneous information concerning patterns of bone growth and remodeling can be obtained if convenient, but biologically incorrect superim- position protocols are used. -

ENDOSCOPIC TRANSMAXILLARY APPROACHES to the SKULL BASE Anatomy, Step-By-Step Guide, and Case Examples
ENDOSCOPIC TRANSMAXILLARY APPROACHES TO THE SKULL BASE Anatomy, Step-by-Step Guide, and Case Examples Hasan ZAIDI, Ali ELHADI, Douglas HARDESTY and Andrew S. LITTLE ENDOSCOPIC TRANSMAXILLARY APPROACHES TO THE SKULL BASE Anatomy, Step-by-Step Guide, and Case Examples Hasan ZAIDI,* Ali ELHADI,* Douglas HARDESTY* and Andrew S. LITTLE* *| MD, Barrow Pituitary and Cranial Base Center and the Barrow Neurosurgery Research Laboratory, Phoenix, AZ, USA 4 Endoscopic Transmaxillary Approaches to the Skull Base Illustrations by: Endoscopic Transmaxillary Approaches Mr. Satyen Tripathi to the Skull Base – Anatomy, Step-by-Step Guide, www.stillustration.com and Case Examples E-mail: [email protected] Hasan Zaidi,* Ali Elhadi,* Douglas Hardesty* and Andrew S. Little* * | MD, Barrow Pituitary and Cranial Base Center and the Barrow Neurosurgery Research Laboratory, Phoenix, AZ, USA Correspondence address of the author: Andrew S. Little, MD c/o Neuroscience Publications; Barrow Neurological Institute St. Joseph’s Hospital and Medical Center 350 W. Thomas Road; Phoenix, AZ 85013, USA Important notes: Phone: +1 (602) 406.3593; Fax: +1 (602) 406.4104 E-mail: [email protected] Medical knowledge is ever changing. As new research and clinical experience broaden our knowledge, changes in treatment and therapy may be required. The authors and editors of the material herein have consulted sources believed to be reliable in their efforts to provide All rights reserved. information that is complete and in accord with the standards 1st Edition accept ed at the time of publication. However, in view of the possibili ty ® of human error by the authors, editors, or publisher, or changes © 2017 GmbH in medical knowledge, neither the authors, editors, publisher, nor P.O. -

Posterior Superior Alveolar Nerves Contribute to Sensation in the Anterior Teeth
Posterior superior alveolar nerves contribute to sensation in the anterior teeth Sanako Makishi Niigata University Mikako Tanaka Meirin College Taichi Kobayashi Niigata University Ray Tanaka University of Hong Kong Takafumi Hayashi Niigata University Hayato Ohshima ( [email protected] ) Niigata University Research Article Keywords: aged, cadaver, histological techniques, three-dimensional imaging, maxillary sinus, X-ray computed tomography Posted Date: December 18th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-123843/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/14 Abstract There is no available data on the occurrence rate of a converged alveolar canal, the detailed three- dimensional (3D) courses of alveolar canals/grooves (ACGs), or the contribution of each superior alveolar nerve to each area in the maxilla. This study aimed to clarify the 3D courses of ACGs, the relationship between ACGs and superior alveolar nerves, and the contribution of posterior superior alveolar nerves (PSANs) using computed tomography (CT) with histological analysis. During the gross anatomy course at Niigata University, we investigated nine human cadavers. All anterior and posterior ACGs converged into the common alveolar canal, which contained blood vessels and several nerve bundles surrounded by perineurium, located at the nasal oor near the pyriform aperture. Histometrical analysis claried that 16.3% of the nerve bundles in this canal were derived from PSANs, and 67% of the bundles were dispersed while they coursed down to the nasal oor. There seems to be no relationship between the density of nerve bundles in the canal and the number of remaining anterior teeth. -
Selection Criteria for the Use of Radiology in Dental Implantology with Emphasis on Cone Beam Computed Tomography Donald A
Vol. 113 No. 6 June 2012 Position statement of the American Academy of Oral and Maxillofacial Radiology on selection criteria for the use of radiology in dental implantology with emphasis on cone beam computed tomography Donald A. Tyndall, DDS, MSPH, PhD,a Jeffery B. Price, DDS, MS,b Sotirios Tetradis, DDS, PhD,c Scott D. Ganz, DMD,d Charles Hildebolt, DDS, PhD,e and William C. Scarfe, BDS, MSf A Position Paper Subcommittee of the American Academy of Oral and Maxillofacial Radiology (AAOMR) reviewed the literature since the original position statement on selection criteria for radiology in dental implantology, published in 2000. All current planar modalities, including intraoral, panoramic, and cephalometric, as well as cone beam computed tomography (CBCT) are discussed, along with radiation dosimetry and anatomy considerations. We provide research- based, consensus-derived clinical guidance for practitioners on the appropriate use of specific imaging modalities in dental implant treatment planning. Specifically, the AAOMR recommends that cross-sectional imaging be used for the assessment of all dental implant sites and that CBCT is the imaging method of choice for gaining this information. This document will be periodically revised to reflect new evidence. (Oral Surg Oral Med Oral Pathol Oral Radiol 2012;113: 817-826) In 2000, the American Academy of Oral and Maxillofa- use in contemporary dental practice; these included cial Radiology (AAOMR) published a position paper on practitioner responsibilities, the requirement for docu- the role of imaging in dental-implant treatment planning.1 mentation, and the need for radiation-dose and quality- They state, “After reviewing the current literature, the assurance optimization.