Palaeoneurological Clues to the Evolution of Defining Mammalian Soft Tissue Traits Received: 27 January 2016 J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Para Nasal Sinusitis : a Problem for the General Practitioner
University of Nebraska Medical Center DigitalCommons@UNMC MD Theses Special Collections 5-1-1933 Para nasal sinusitis : a problem for the general practitioner Robert James Ralston University of Nebraska Medical Center This manuscript is historical in nature and may not reflect current medical research and practice. Search PubMed for current research. Follow this and additional works at: https://digitalcommons.unmc.edu/mdtheses Recommended Citation Ralston, Robert James, "Para nasal sinusitis : a problem for the general practitioner" (1933). MD Theses. 283. https://digitalcommons.unmc.edu/mdtheses/283 This Thesis is brought to you for free and open access by the Special Collections at DigitalCommons@UNMC. It has been accepted for inclusion in MD Theses by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. PARA-NASAL SINUSITIS A PROBLEM FOR THE GENERAL PRACTITIONER by Rooert James Ralston, B. Sc. Presented to the Faculty of The College of Medicine in the University of Nebraska in Partial Fulfillment of the Requirements - for the Degree of Doctor of Medicine 1933 Omaha, Nebraska TABLE O.F' CONTENTS Pages I Introductory and Historioal 1 - 2 II Anatomioal Discussion of Nose and Accessory Nasal Sinuses 3 - 16 III Etiology and Inoidence of Paranasal Sinus Infection 17 - 20 IV Pathology of Paranasal Sinusitis 21 - 23 V Sinus Infection in Relation to Systemic Disease 24 - 28 VI Symptoms and Diagnosis of Paranasal Sinusitis 29 - 38 VII Treatment of Paranasal Sinusitis 39 - 45 VIII Case Histories 46 - 55 IX Conclusion 56 X Bibliography 1 INTRODUOTORY AND HISTORICAL The paranasal, or accessory sinuses of the nose, are as sociated with the general health of the individual just as closely as are the tonsils, the teeth, the gall bladder, or any other of the commonly accepted foci of infection in the human body. -

Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Gondwana Vertebrate Faunas of India: Their Diversity and Intercontinental Relationships
438 Article 438 by Saswati Bandyopadhyay1* and Sanghamitra Ray2 Gondwana Vertebrate Faunas of India: Their Diversity and Intercontinental Relationships 1Geological Studies Unit, Indian Statistical Institute, 203 B. T. Road, Kolkata 700108, India; email: [email protected] 2Department of Geology and Geophysics, Indian Institute of Technology, Kharagpur 721302, India; email: [email protected] *Corresponding author (Received : 23/12/2018; Revised accepted : 11/09/2019) https://doi.org/10.18814/epiiugs/2020/020028 The twelve Gondwanan stratigraphic horizons of many extant lineages, producing highly diverse terrestrial vertebrates India have yielded varied vertebrate fossils. The oldest in the vacant niches created throughout the world due to the end- Permian extinction event. Diapsids diversified rapidly by the Middle fossil record is the Endothiodon-dominated multitaxic Triassic in to many communities of continental tetrapods, whereas Kundaram fauna, which correlates the Kundaram the non-mammalian synapsids became a minor components for the Formation with several other coeval Late Permian remainder of the Mesozoic Era. The Gondwana basins of peninsular horizons of South Africa, Zambia, Tanzania, India (Fig. 1A) aptly exemplify the diverse vertebrate faunas found Mozambique, Malawi, Madagascar and Brazil. The from the Late Palaeozoic and Mesozoic. During the last few decades much emphasis was given on explorations and excavations of Permian-Triassic transition in India is marked by vertebrate fossils in these basins which have yielded many new fossil distinct taxonomic shift and faunal characteristics and vertebrates, significant both in numbers and diversity of genera, and represented by small-sized holdover fauna of the providing information on their taphonomy, taxonomy, phylogeny, Early Triassic Panchet and Kamthi fauna. -

Chapter 2 Implants and Oral Anatomy
Chapter 2 Implants and oral anatomy Associate Professor of Maxillofacial Anatomy Section, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University Tatsuo Terashima In recent years, the development of new materials and improvements in the operative methods used for implants have led to remarkable progress in the field of dental surgery. These methods have been applied widely in clinical practice. The development of computerized medical imaging technologies such as X-ray computed tomography have allowed detailed 3D-analysis of medical conditions, resulting in a dramatic improvement in the success rates of operative intervention. For treatment with a dental implant to be successful, it is however critical to have full knowledge and understanding of the fundamental anatomical structures of the oral and maxillofacial regions. In addition, it is necessary to understand variations in the topographic and anatomical structures among individuals, with age, and with pathological conditions. This chapter will discuss the basic structure of the oral cavity in relation to implant treatment. I. Osteology of the oral area The oral cavity is composed of the maxilla that is in contact with the cranial bone, palatine bone, the mobile mandible, and the hyoid bone. The maxilla and the palatine bones articulate with the cranial bone. The mandible articulates with the temporal bone through the temporomandibular joint (TMJ). The hyoid bone is suspended from the cranium and the mandible by the suprahyoid and infrahyoid muscles. The formation of the basis of the oral cavity by these bones and the associated muscles makes it possible for the oral cavity to perform its various functions. -
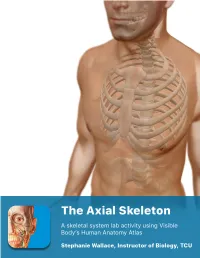
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -

A Non-Mammaliaform Cynodont from the Upper Triassic of South Africa: a Therapsid Lazarus Taxon?
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Wits Institutional Repository on DSPACE A non-mammaliaform cynodont from the Upper Triassic of South Africa: a therapsid Lazarus taxon? Fernando Abdala1*, Ross Damiani2, Adam Yates1 & Johann Neveling3 1Bernard Price Institute for Palaeontological Research, School of Geosciences, University of the Witwatersrand, Private Bag 3, WITS, 2050 South Africa 2Staatliches Museum für Naturkunde Stuttgart, Rosenstein 1, D-70191, Stuttgart, Germany 3Council for Geoscience, Private Bag X112, Pretoria, 0001 South Africa Received 20 January 2006. Accepted 10 January 2007 The tetrapod record of the ‘Stormberg Group’, including the Lower Elliot Formation, in the South African Karoo is widely dominated by archosaurian reptiles, contrasting with the therapsid dominion of the subjacent Beaufort Group. The only therapsids represented by skeletal remains in the Upper Triassic Lower Elliot Formation are the large traversodontid cynodont Scalenodontoides macrodontes and the recently described tritheledontid cynodont Elliotherium kersteni. Here we present a fragmentary lower jaw that provides evidence of a third type of cynodont for the Upper Triassic of South Africa. The fossil is tentatively assigned to the Diademodontidae. The latter representative of this family is known from the Late Anisian, and its tentative record in the Norian Lower Elliot Formation, if confirmed, will represent a case of Lazarus taxon. Thus, Diademodontidae apparently disappeared from the fossil record by the end of the Anisian and then reappeared in the Norian of South Africa, a stratigraphic interval of some 21 million years. This new cynodont record, together with the recently described Tritheledontidae, show that cynodonts are now the second most diverse tetrapod group in the Lower Elliot fauna. -

Osteohistology of Late Triassic Prozostrodontian Cynodonts from Brazil
Osteohistology of Late Triassic prozostrodontian cynodonts from Brazil Jennifer Botha-Brink1,2, Marina Bento Soares3 and Agustín G. Martinelli3 1 Department of Karoo Palaeontology, National Museum, Bloemfontein, South Africa 2 Department of Zoology and Entomology, University of the Free State, Bloemfontein, South Africa 3 Departamento de Paleontologia e Estratigrafia, Instituto de Geociências, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil ABSTRACT The Prozostrodontia includes a group of Late Triassic-Early Cretaceous eucynodonts plus the clade Mammaliaformes, in which Mammalia is nested. Analysing their growth patterns is thus important for understanding the evolution of mammalian life histories. Obtaining material for osteohistological analysis is difficult due to the rare and delicate nature of most of the prozostrodontian taxa, much of which comprises mostly of crania or sometimes even only teeth. Here we present a rare opportunity to observe the osteohistology of several postcranial elements of the basal prozostrodontid Prozostrodon brasiliensis, the tritheledontid Irajatherium hernandezi, and the brasilodontids Brasilodon quadrangularis and Brasilitherium riograndensis from the Late Triassic of Brazil (Santa Maria Supersequence). Prozostrodon and Irajatherium reveal similar growth patterns of rapid early growth with annual interruptions later in ontogeny. These interruptions are associated with wide zones of slow growing bone tissue. Brasilodon and Brasilitherium exhibit a mixture of woven-fibered bone tissue and slower growing parallel-fibered and lamellar bone. The slower growing bone tissues are present even during early ontogeny. The relatively slower growth in Brasilodon and Brasilitherium may be related to their small body size compared to Prozostrodon and Irajatherium. These brasilodontids also exhibit osteohistological similarities with the Late Triassic/Early Jurassic mammaliaform Morganucodon and the Late Cretaceous multituberculate mammals Kryptobaatar and Nemegtbaatar. -

Physical and Environmental Drivers of Paleozoic Tetrapod Dispersal Across Pangaea
ARTICLE https://doi.org/10.1038/s41467-018-07623-x OPEN Physical and environmental drivers of Paleozoic tetrapod dispersal across Pangaea Neil Brocklehurst1,2, Emma M. Dunne3, Daniel D. Cashmore3 &Jӧrg Frӧbisch2,4 The Carboniferous and Permian were crucial intervals in the establishment of terrestrial ecosystems, which occurred alongside substantial environmental and climate changes throughout the globe, as well as the final assembly of the supercontinent of Pangaea. The fl 1234567890():,; in uence of these changes on tetrapod biogeography is highly contentious, with some authors suggesting a cosmopolitan fauna resulting from a lack of barriers, and some iden- tifying provincialism. Here we carry out a detailed historical biogeographic analysis of late Paleozoic tetrapods to study the patterns of dispersal and vicariance. A likelihood-based approach to infer ancestral areas is combined with stochastic mapping to assess rates of vicariance and dispersal. Both the late Carboniferous and the end-Guadalupian are char- acterised by a decrease in dispersal and a vicariance peak in amniotes and amphibians. The first of these shifts is attributed to orogenic activity, the second to increasing climate heterogeneity. 1 Department of Earth Sciences, University of Oxford, South Parks Road, Oxford OX1 3AN, UK. 2 Museum für Naturkunde, Leibniz-Institut für Evolutions- und Biodiversitätsforschung, Invalidenstraße 43, 10115 Berlin, Germany. 3 School of Geography, Earth and Environmental Sciences, University of Birmingham, Birmingham B15 2TT, UK. 4 Institut -

Atlas of the Facial Nerve and Related Structures
Rhoton Yoshioka Atlas of the Facial Nerve Unique Atlas Opens Window and Related Structures Into Facial Nerve Anatomy… Atlas of the Facial Nerve and Related Structures and Related Nerve Facial of the Atlas “His meticulous methods of anatomical dissection and microsurgical techniques helped transform the primitive specialty of neurosurgery into the magnificent surgical discipline that it is today.”— Nobutaka Yoshioka American Association of Neurological Surgeons. Albert L. Rhoton, Jr. Nobutaka Yoshioka, MD, PhD and Albert L. Rhoton, Jr., MD have created an anatomical atlas of astounding precision. An unparalleled teaching tool, this atlas opens a unique window into the anatomical intricacies of complex facial nerves and related structures. An internationally renowned author, educator, brain anatomist, and neurosurgeon, Dr. Rhoton is regarded by colleagues as one of the fathers of modern microscopic neurosurgery. Dr. Yoshioka, an esteemed craniofacial reconstructive surgeon in Japan, mastered this precise dissection technique while undertaking a fellowship at Dr. Rhoton’s microanatomy lab, writing in the preface that within such precision images lies potential for surgical innovation. Special Features • Exquisite color photographs, prepared from carefully dissected latex injected cadavers, reveal anatomy layer by layer with remarkable detail and clarity • An added highlight, 3-D versions of these extraordinary images, are available online in the Thieme MediaCenter • Major sections include intracranial region and skull, upper facial and midfacial region, and lower facial and posterolateral neck region Organized by region, each layered dissection elucidates specific nerves and structures with pinpoint accuracy, providing the clinician with in-depth anatomical insights. Precise clinical explanations accompany each photograph. In tandem, the images and text provide an excellent foundation for understanding the nerves and structures impacted by neurosurgical-related pathologies as well as other conditions and injuries. -

V53.2018.PSSA Abstracts
tropical to warm temperate palaeoenvironments, principally in Laurussia and the northern continental blocks. By contrast, the Waterloo Farm Lagerstätte, from which Archaeopteris notosaria was described, was situated in southern Gondwana at high palaeolatitude (currently reconstructed as being within the Devonian Antarctic Circle). This was the first evidence that cosmopolitan lowland forests had achieved a truly global distribution by the end of the Devonian. The environmental implications of this vegetative explosion have been explored by subsequent authors, particularly with regard to climate change and possible causes of the End Devonian Mass Extinction. The original description relied on portions of leafy ‘fronds’, and a single poorly preserved fertile fragment. New material has been provided by excavation of a stratigraphically lower site discovered during roadworks in the mid portion of the Witpoort Formation. Not only does this provide a slightly earlier record for the arrival of Archaeopteris in southern Africa but is also significant in that the new material provides additional anatomical information. It includes impres - sions of two whole ‘fronds’, one infertile and one fertile, as well as a fragment in which the structure of the fertile cones is clearly apparent, revealing the type material to have been incomplete. Bone lesion in a gorgonopsian fossil from the Permian of Zambia: periosteal reaction in a premammalian synapsid Kyle Kato 1, Elizabeth Rega 2, Christian Sidor 3, Adam K. Huttenlocker 1* 1Department of Integrative Anatomical Sciences, Keck School of Medicine, University of Southern California, Los Angeles, California, U.S.A. 2College of Osteopathic Medicine of the Pacific and the Graduate College of Biomedical Sciences, Western University of Health Sciences, Pomona, California, U.S.A. -

Variability of the Parietal Foramen and the Evolution of the Pineal Eye in South African Permo-Triassic Eutheriodont Therapsids
The sixth sense in mammalian forerunners: Variability of the parietal foramen and the evolution of the pineal eye in South African Permo-Triassic eutheriodont therapsids JULIEN BENOIT, FERNANDO ABDALA, PAUL R. MANGER, and BRUCE S. RUBIDGE Benoit, J., Abdala, F., Manger, P.R., and Rubidge, B.S. 2016. The sixth sense in mammalian forerunners: Variability of the parietal foramen and the evolution of the pineal eye in South African Permo-Triassic eutheriodont therapsids. Acta Palaeontologica Polonica 61 (4): 777–789. In some extant ectotherms, the third eye (or pineal eye) is a photosensitive organ located in the parietal foramen on the midline of the skull roof. The pineal eye sends information regarding exposure to sunlight to the pineal complex, a region of the brain devoted to the regulation of body temperature, reproductive synchrony, and biological rhythms. The parietal foramen is absent in mammals but present in most of the closest extinct relatives of mammals, the Therapsida. A broad ranging survey of the occurrence and size of the parietal foramen in different South African therapsid taxa demonstrates that through time the parietal foramen tends, in a convergent manner, to become smaller and is absent more frequently in eutherocephalians (Akidnognathiidae, Whaitsiidae, and Baurioidea) and non-mammaliaform eucynodonts. Among the latter, the Probainognathia, the lineage leading to mammaliaforms, are the only one to achieve the complete loss of the parietal foramen. These results suggest a gradual and convergent loss of the photoreceptive function of the pineal organ and degeneration of the third eye. Given the role of the pineal organ to achieve fine-tuned thermoregulation in ecto- therms (i.e., “cold-blooded” vertebrates), the gradual loss of the parietal foramen through time in the Karoo stratigraphic succession may be correlated with the transition from a mesothermic metabolism to a high metabolic rate (endothermy) in mammalian ancestry. -

Origin and Relationships of the Ictidosauria to Non-Mammalian
Historical Biology An International Journal of Paleobiology ISSN: 0891-2963 (Print) 1029-2381 (Online) Journal homepage: http://www.tandfonline.com/loi/ghbi20 Origin and relationships of the Ictidosauria to non- mammalian cynodonts and mammals José F. Bonaparte & A. W. Crompton To cite this article: José F. Bonaparte & A. W. Crompton (2017): Origin and relationships of the Ictidosauria to non-mammalian cynodonts and mammals, Historical Biology, DOI: 10.1080/08912963.2017.1329911 To link to this article: http://dx.doi.org/10.1080/08912963.2017.1329911 Published online: 23 Jun 2017. Submit your article to this journal Article views: 53 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ghbi20 Download by: [Smithsonian Astrophysics Observatory] Date: 06 November 2017, At: 13:06 HISTORICAL BIOLOGY, 2017 https://doi.org/10.1080/08912963.2017.1329911 Origin and relationships of the Ictidosauria to non-mammalian cynodonts and mammals José F. Bonapartea and A. W. Cromptonb aMuseo Municipal de C. Naturales “C. Ameghino”, Mercedes, Argentina; bMuseum of Comparative Zoology, Harvard University, Cambridge, MA, USA ABSTRACT ARTICLE HISTORY Ictidosaurian genera are allocated to two families, Tritheledontidae and Therioherpetidae. This paper Received 19 December 2016 provides a diagnosis for Ictidosauria. The previously named family Brasilodontidae is shown to be a Accepted 30 April 2017 junior synonym of a family, Therioherpetidae. It is concluded that Ictidosauria originated from Late KEYWORDS Permian procynosuchid non-mammalian cynodonts rather than from Middle Triassic probainognathid Mammalian origins; non-mammalian cynodonts. The structure of the skull and jaws of a derived traversodontid Ischignathus Ictidosauria; Tritylodontia; sudamericanus from the early Late Triassic of Argentina supports an earlier view that tritylodontids are Brasilitherium more closely related to traversodontid than probainognathid non-mammalian cynodonts.