United States Patent 0 " 1C6 Patented Dec
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Pp-03-25-New Dots.Qxd 10/23/02 2:41 PM Page 778
pp-03-25-new dots.qxd 10/23/02 2:41 PM Page 778 778 PRAESODYMIUM PRAESODYMIUM [7440–10–0] Symbol Pr; atomic number 59; atomic weight 140.908; a lanthanide–series rare earth element; belongs to the cerium group of rare earths; electron con- figuration [Xe] 4f36s2; partially filled f subshell; valence states +3, +4; most 3+ stable oxidation state +3; electrode potential E°/V (aq) for Pr + 3e¯ ↔ Pr is –2.35 V; atomic radius 1.828 Å; first ionization potential 5.46 eV; one natu- rally–occurring isotope, Pr–141; twenty–nine artificial radioactive isotopes known in the mass range 124, 126–140 and 142–154; the longest–lived isotope Pr–143, t1/2 13.57 day, and the shortest–lived isotope Pr–124, t1/2 1.2 second. History, Occurrence, and Uses Mosander extracted from the mineral lanthana a rare earth fraction, named didymia in 1841. In 1879, Boisbaudran separated a rare earth oxide called samaria (samarium oxide) from the didymia fraction obtained from the mineral samarskite. Soon after that in 1885, Baron Auer von Welsbach iso- lated two other rare earths from didymia. He named them as praseodymia (green twin) and neodymia (new twin) after their source didymia (twin). The name praseodymium finally was assigned to this new element, derived from the two Greek words, prasios meaning green and didymos meaning twin. Praseodymium occurs in nature associated with other rare earths in a rel- atively high abundance. It is more abundant than some common metals such as silver, gold, or antimony. The average concentration of this metal in the earth’s crust is estimated to be 8.2 mg/kg. -

The Preparation and Reactions of the Lower Chlorides and Oxychlorides of Silicon Joseph Bradley Quig Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1926 The preparation and reactions of the lower chlorides and oxychlorides of silicon Joseph Bradley Quig Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Inorganic Chemistry Commons Recommended Citation Quig, Joseph Bradley, "The preparation and reactions of the lower chlorides and oxychlorides of silicon " (1926). Retrospective Theses and Dissertations. 14278. https://lib.dr.iastate.edu/rtd/14278 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UlVli films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and impiroper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overiaps. -

IODINE Its Properties and Technical Applications
IODINE Its Properties and Technical Applications CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York IODINE Its Properties and Technical Applications ¡¡iiHiüíiüüiütitittüHiiUitítHiiiittiíU CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York 1951 Copyright, 1951, by Chilean Iodine Educational Bureau, Inc. Printed in U.S.A. Contents Page Foreword v I—Chemistry of Iodine and Its Compounds 1 A Short History of Iodine 1 The Occurrence and Production of Iodine ....... 3 The Properties of Iodine 4 Solid Iodine 4 Liquid Iodine 5 Iodine Vapor and Gas 6 Chemical Properties 6 Inorganic Compounds of Iodine 8 Compounds of Electropositive Iodine 8 Compounds with Other Halogens 8 The Polyhalides 9 Hydrogen Iodide 1,0 Inorganic Iodides 10 Physical Properties 10 Chemical Properties 12 Complex Iodides .13 The Oxides of Iodine . 14 Iodic Acid and the Iodates 15 Periodic Acid and the Periodates 15 Reactions of Iodine and Its Inorganic Compounds With Organic Compounds 17 Iodine . 17 Iodine Halides 18 Hydrogen Iodide 19 Inorganic Iodides 19 Periodic and Iodic Acids 21 The Organic Iodo Compounds 22 Organic Compounds of Polyvalent Iodine 25 The lodoso Compounds 25 The Iodoxy Compounds 26 The Iodyl Compounds 26 The Iodonium Salts 27 Heterocyclic Iodine Compounds 30 Bibliography 31 II—Applications of Iodine and Its Compounds 35 Iodine in Organic Chemistry 35 Iodine and Its Compounds at Catalysts 35 Exchange Catalysis 35 Halogenation 38 Isomerization 38 Dehydration 39 III Page Acylation 41 Carbón Monoxide (and Nitric Oxide) Additions ... 42 Reactions with Oxygen 42 Homogeneous Pyrolysis 43 Iodine as an Inhibitor 44 Other Applications 44 Iodine and Its Compounds as Process Reagents ... -

(12) Patent Application Publication (10) Pub. No.: US 2016/0115593 A1 KUCHENBESER Et Al
US 2016O115593A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0115593 A1 KUCHENBESER et al. (43) Pub. Date: Apr. 28, 2016 (54) AMINO(IODO)SILANE PRECURSORS FOR HOIL 2/3 II (2006.01) ALDACVD SILICON-CONTAINING FILM C23C I6/34 (2006.01) APPLICATIONS AND METHODS OF USING C23C I6/56 (2006.01) THE SAME (52) U.S. Cl. CPC ......... C23C 16/45553 (2013.01); C23C 16/345 (71) Applicant: American Air Liquide, Inc., Fremont, (2013.01); C23C I6/45536 (2013.01); C23C CA (US) I6/56 (2013.01); HOIL 21/31 III (2013.01); HOIL 21/02211 (2013.01); HOIL 21/02274 (72) Inventors: Glenn KUCHENBEISER, Newark, DE (2013.01); HOIL 2L/0228 (2013.01); HOIL (US); Bastien LEFEVRE, Wilmington, 21/0217 (2013.01) DE (US) (21) Appl. No.: 14/984,866 (57) ABSTRACT (22) Filed: Dec. 30, 2015 Publication Classification Disclosed are amino(iodo)silane precursors, methods of syn thesizing the same, and methods of using the same to deposit (51) Int. Cl. silicon-containing films using vapor deposition processes. C23C I6/455 (2006.01) The disclosed amino(iodo)silane precursors include SiHI(N HOIL 21/02 (2006.01) (iPr)) or SiHI(N(iBu)). Patent Application Publication Apr. 28, 2016 Sheet 1 of 6 US 2016/O115593 A1 FG Patent Application Publication Apr. 28, 2016 Sheet 2 of 6 US 2016/O115593 A1 100 T - - - - 90 - SiH(NMe2)2 atmoc 44mg 80 - - - - SiH(NMe2)2 closed cup 70 - 50 - 30 - 10 - O 1 OO 2OO 300 400 500 Temperature (°C) FIG. 3 - - SiH2(NEt2) atmoc 41 mg SiH2(NEt2) closed Cup 22mg s s cus s 9 CD 8 300 Temperature (°C) FIG. -

Dec. 12, 1961 H
Dec. 12, 1961 H. W. LNG 3,012,861 PRODUCTION OF SILICON Filed Jan. 15, 1960 zzzzz INVENTOR HARRY W. LING ATTORNEY 3,012,861 United States Patent Office Patented Dec. 12, 1961 1. 2 3,012,861 . Referring now to this drawing, there is shown a ver PRODUCTION OF SELICON tically disposed cylindrical or tubular reactor 1 with a Harry W. Ling, Wilmington, Del assignor to E. I. du conical bottom 3 provided with inlet 4 for entrance of Pont de Nemours and Company, Wilmington, Dei, a reactant fluidizing gas. The reactor 1 is sufficiently high corporation of Delaware to provide for a 2-3 times expansion of the bed 6 and also Filed Jan. 15, 1960, Ser. No. 3,708. a disengaging space 7 above the bed 6 to reduce solids 9. Claims. (C. 23-223.5) blowover to a minimum. A valved inlet 8 is provided for introduction of silicon bed particles. The reaction This invention relates to the production of elemental by-product gases are removed from the reactor through silicon. More particularly, it relates to a new and im 10 the outlet 9 and cyclone 13 which can be kept hot by a proved method of thermally decomposing volatile silicon furnace (not shown). These gases then pass to a con compounds. densing system 10 and cold trap (not shown) through Silicon can be produced by thermally decomposing sili which the vacuum pumping system (not shown) is con con iodide at a reduced pressure -on an electrically nected. The reactor 1 is maintained at the desired reactor heated, incandescent metal filament. -

In Presenting This Dissertation As a Partial Fulfillment of The
In presenting this dissertation as a partial fulfillment of the requirements for an advanced degree from the Georgia Institute of Tech nology, I agree that the Library of the Institution shall make it avail able for inspection and circulation in accordance with its regulations governing materials of this type. I agree that permission to copy from, or to publish from, this dissertation may be granted by the professor under -whose direction it was written, when such copying or publication is solely for scholarly purposes and does not involve potential financial gain. It is understood that any copying from, or publication of, thie dissertation which involves potential financial gain will not be allowed without written permission. Thomas Grindley AN EVALUATION OF U. S. PATENT LITERATURE PERTAINING TO THE PRODUCTION OF TITANIUM A THESIS Presented to the Faculty of the Graduate Division Georgia Institute of Technology In Partial Fulfillment of the Requirements for the Degree Master of Science in Chemical Engineering By Thomas Grindley June 19£6 pf -* ^r0" > AN EVALUATION OF U.S. PATENT LITERATURE PERTAINING TO THE PRODUCTION OF TITANIUM Approved: n ir~ Date Approved by Chairman: >^^, 3 <••/s"Z ii ACKNOWLEDGMENT The author wishes to thank most heartily, certain members of the staff of the Price-Gilbert Library for their patience in listening to his requests and for their kind assistance in helping him to find material for this thesis. The author felt very much at home in the Library. TABLE OF CONTENTS Chapter Page X. INTRODUCTION 1 H. HISTORICAL Development of Extraction Process Commerical History in . PRESENT PRACTICE IN TITANIUM EXTRACTION 10 Chlorination of the Ore Purification of Crude Titanium Tetrachloride Magnesium Reduction Removal of the Titanium Sponge Vacuum Distillation of Magnesium and Magnesium Chloride Leaching Melting and Ingot Formation IV. -

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

INTI071 GHS EU English
INTI071 - TITANIUM TETRAIODIDE, 99.99% on metals basis TITANIUM TETRAIODIDE, 99.99% on metals basis Safety Data Sheet INTI071 Date of issue: 02/04/2015 Revision date: 24/05/2018 Version: 1.2 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Substance Physical state : Solid Substance name : TITANIUM TETRAIODIDE, 99.99% on metals basis Product code : INTI071 Formula : I4Ti2 Synonyms : TETRAIODOTITANIUM Chemical family : METAL COMPOUND 1.2. Relevant identified uses of the substance or mixture and uses advised against 1.2.1. Relevant identified uses Use of the substance/mixture : Chemical intermediate 1.2.2. Uses advised against No additional information available 1.3. Details of the supplier of the safety data sheet GELEST, INC. 11 East Steel Road Morrisville, PA 19067 USA T 215-547-1015 - F 215-547-2484 - (M-F): 8:00 AM - 5:30 PM EST [email protected] - www.gelest.com GELEST INC. Fritz-Klatte-Strasse 8 65933 Frankfurt Germany T +49 (0) 69 3535106-500 - F +49 (0) 69 3535106-501 - (M-F): 8:00 AM - 4:00 PM [email protected] - www.gelestde.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 (USA); +1 703-527-3887 (International) SECTION 2: Hazards identification 2.1. Classification of the substance or mixture Classification according to Regulation (EC) No. 1272/2008 [CLP] Skin corrosion/irritation, Category 1B H314 Serious eye damage/eye irritation, Category 1 H318 Specific target organ toxicity — Single exposure, Category 3, H335 Respiratory tract irritation Full text of H statements : see section 16 Adverse physicochemical, human health and environmental effects No additional information available 2.2. -

A Study of the Addition Compounds of Titanium Tetrabromide and Titanium Tetraiodide with 1,4-Dioxane, Tetrahydrofuran, and Tetrahydrqpyran
A STUDY OF THE ADDITION COMPOUNDS OF TITANIUM TETRABROMIDE AND TITANIUM TETRAIODIDE WITH 1,4-DIOXANE, TETRAHYDROFURAN, AND TETRAHYDRQPYRAN DISSERTATION Presented in Partial Fulfillment of tha Requirements for the Degree Doctor of Philosphy in the Graduate School of The Ohio State University Robert Fredrick Rolsten, B.S. The Ohio State University 1955 Approved by: D r ACKNOWLEDGEMENTS The author wishes to express appreciation to Dr. Harry H. Sisler for his continued personal interest and guidance throughout the course of the investigation. Appreciation is also extended to Dr. Edward D. Loughran for his cooperation in the use of the infrared spectro photometer, to Dr. Preston M. Harris and Mr. Herbert W. Newkirk for contributing to the interpretation of the X-Ray data. The arrangement between Battelle Memorial Institute and the Graduate School of The Ohio State University with regard to tuition is gratefully acknowledged. I fully recognize the sacrifice my children have made in tolerating my necessary absence while attending school and which resulted in my failing of fatherly obligations. I sincerely hope the means has not destroyed the end. ii TABLE OF CONTENTS Page ACKNOWLEDGEMENTS ......................................... ...... ii LIST OF ILLUSTRATIONS .......................................... iv LIST OF TABIES ............................................. viii I. INTRODUCTION .................................. 1 II. HISTORICAL ............................................... 3 III. PREPARATION AND PURIFICATION OF REAGENTS ............... -

WO 2013/089962 Al 20 June 2013 (20.06.2013) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/089962 Al 20 June 2013 (20.06.2013) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every B01J 31/04 (2006.01) B01J 31/18 (2006.01) kind of national protection available): AE, AG, AL, AM, B01J 31/14 (2006.01) B01J 31/22 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US20 12/065285 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 15 November 2012 (15.1 1.2012) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (25) Filing Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (26) Publication Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 13/323,328 12 December 201 1 (12. 12.201 1) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): CHEV¬ GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, RON PHILLIPS CHEMICAL COMPANY LP UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, [US/US]; 10001 Six Pines Drive, The Woodlands, Texas TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, 77380 (US). -

Silicon Semiconductor Technology
Runyan TEXAS INSTRUMENTS INCORPORATED Semiconductor·Components Division EXAS INSTRUMENTS INCORPORATED Silicon Semiconductor Technology W. R. Runyan Texas Instruments Electronics Series McGraw·HiII McGraw-Hili Beok Company 54276 Silicon Semiconductor Technology The Engineering Staff of Texas Instruments Incorporated • TRANSISTOR CIRCUIT DESIGN Runyan • SILICON SEMICONDUCTOR TECHNOLOGY Sevin • FIELD-EFFECT TRANSISTORS Silicon Semiconductor Technology w. R. Runyan Semiconductor Research and Development Laboratory Texas Instruments Incorporated McGRAW-HILL BOOK COMPANY New York San Francisco Toronto London Sydney SILICON SEMICONDUCTOR TECHNOLOGY Copyright © 1965 by Texas Instruments Incorporated. All Rights Reserved. Printed in the United States of America. This book, or parts thereof, may not be reproduced in any form without permission of Texas Instru ments Incorporated. Library of Congress Catalog Card Number 64-24607. Information contained in this book is believed to be accurate and reliable. However, responsibility is assumed neither for its use nor for any infringement of patents or rights of others which may result from its use. No license is granted by implication or otherwise under any patent or patent right of Texas Instruments or others. 54276 345678 9-MP-9 8 7 6 Preface The purposes of this book are to provide in a single reference the properties of silicon important to those who would use it as a semiconductor and to discuss at length several of the more important semiconductor technologies, such as crystal growing and diffusion. It had its beginning in a set of notes I began compiling shortly after starting work in the semiconductor industry. Since most of my activ ities were with silicon, these notes were restricted to information pertaining to that material. -
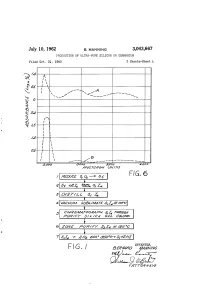
N FIG. I Azawad "Wiiw4
July 10, 1962 B, MANNING 3,043,667 PRODUCTION OF ULTRA-PURE SELICON OR GERMANIUM Filed Oct. 31, 1960 3 Sheets-Sheet N 220 eaded sao 162 o o F.G. 6 3. 1. | 6ao.4%726/24a/ s. 77%WG/ ape/a/ay1 9/4 /64 Gaa. 404A2/V FIG. I azawad "WiiW4 B%0/.-- 2.2 77(42.45 Oag A/AX-S July 10, 1962 B. MANNING 3,043,667 PRODUCTION OF ULTRA-PURE SILICON OR GERMANIUM Filed Oct. 31, 1960 3. Sheets-Sheet 2 in SS INVENTOR, ABAA/WAAA M/4///////4 2AMWAS July 10, 1962 B, MANNING 3,043,667 PRODUCTION OF ULTRA-PURE SILICON OR GERMANIUM Filed Oct. 3l, 1960 3. Sheets-Sheet 3 2 4. 8 as7 s i.\ \ INVENTOR. AtAA/VAAA) M/4//W/WG B 'cC-4- 2. 92 - . Z 775"/0231. a Maas 3,043,667 United States Patent Office Patiented July 10, 1962 2 adsorbing impurities from the tetrahalide by passing a 3,043,667 solution thereof through purified silica gel, recovering PRODUCTION OF ULTRA-PURE SELICON the tetrahalide from the solution, Zone purifying the OR GERMANUM tetrahalide by passing a mass thereof slowly past alternate Bernard Manning, Waltham, Mass, assignor to the melting and cooling zones, and reducing the tetrahalide to United States of America as represented by the Secre elemental silicon or germanium by passing hydrogen gas tary of the Air Force into a retort containing the halide heated to an elevated Filed Oct. 3, 1960, Ser. No. 66,369 temperature, said gas and halide vapor passing into a 2 Claims.