A Mitochondrial Genome of Rhyparochromidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Methods and Work Profile
REVIEW OF THE KNOWN AND POTENTIAL BIODIVERSITY IMPACTS OF PHYTOPHTHORA AND THE LIKELY IMPACT ON ECOSYSTEM SERVICES JANUARY 2011 Simon Conyers Kate Somerwill Carmel Ramwell John Hughes Ruth Laybourn Naomi Jones Food and Environment Research Agency Sand Hutton, York, YO41 1LZ 2 CONTENTS Executive Summary .......................................................................................................................... 8 1. Introduction ............................................................................................................ 13 1.1 Background ........................................................................................................................ 13 1.2 Objectives .......................................................................................................................... 15 2. Review of the potential impacts on species of higher trophic groups .................... 16 2.1 Introduction ........................................................................................................................ 16 2.2 Methods ............................................................................................................................. 16 2.3 Results ............................................................................................................................... 17 2.4 Discussion .......................................................................................................................... 44 3. Review of the potential impacts on ecosystem services ....................................... -

The Influence of Prairie Restoration on Hemiptera
CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Thesis Submitted to The College of Arts and Sciences of the UNIVERSITY OF DAYTON In Partial Fulfillment of the Requirements for The Degree of Master of Science in Biology By Stephanie Kay Gunter, B.A. Dayton, Ohio August 2021 CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Name: Gunter, Stephanie Kay APPROVED BY: Chelse M. Prather, Ph.D. Faculty Advisor Associate Professor Department of Biology Ryan W. McEwan, Ph.D. Committee Member Associate Professor Department of Biology Mark G. Nielsen Ph.D. Committee Member Associate Professor Department of Biology ii © Copyright by Stephanie Kay Gunter All rights reserved 2021 iii ABSTRACT CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Name: Gunter, Stephanie Kay University of Dayton Advisor: Dr. Chelse M. Prather Ohio historically hosted a patchwork of tallgrass prairies, which provided habitat for native species and prevented erosion. As these vulnerable habitats have declined in the last 200 years due to increased human land use, restorations of these ecosystems have increased, and it is important to evaluate their success. The Hemiptera (true bugs) are an abundant and varied order of insects including leafhoppers, aphids, cicadas, stink bugs, and more. They play important roles in grassland ecosystems, feeding on plant sap and providing prey to predators. Hemipteran abundance and composition can respond to grassland restorations, age of restoration, and size and isolation of habitat. -

Insects & Spiders of Kanha Tiger Reserve
Some Insects & Spiders of Kanha Tiger Reserve Some by Aniruddha Dhamorikar Insects & Spiders of Kanha Tiger Reserve Aniruddha Dhamorikar 1 2 Study of some Insect orders (Insecta) and Spiders (Arachnida: Araneae) of Kanha Tiger Reserve by The Corbett Foundation Project investigator Aniruddha Dhamorikar Expert advisors Kedar Gore Dr Amol Patwardhan Dr Ashish Tiple Declaration This report is submitted in the fulfillment of the project initiated by The Corbett Foundation under the permission received from the PCCF (Wildlife), Madhya Pradesh, Bhopal, communication code क्रम 車क/ तकनीकी-I / 386 dated January 20, 2014. Kanha Office Admin office Village Baherakhar, P.O. Nikkum 81-88, Atlanta, 8th Floor, 209, Dist Balaghat, Nariman Point, Mumbai, Madhya Pradesh 481116 Maharashtra 400021 Tel.: +91 7636290300 Tel.: +91 22 614666400 [email protected] www.corbettfoundation.org 3 Some Insects and Spiders of Kanha Tiger Reserve by Aniruddha Dhamorikar © The Corbett Foundation. 2015. All rights reserved. No part of this book may be used, reproduced, or transmitted in any form (electronic and in print) for commercial purposes. This book is meant for educational purposes only, and can be reproduced or transmitted electronically or in print with due credit to the author and the publisher. All images are © Aniruddha Dhamorikar unless otherwise mentioned. Image credits (used under Creative Commons): Amol Patwardhan: Mottled emigrant (plate 1.l) Dinesh Valke: Whirligig beetle (plate 10.h) Jeffrey W. Lotz: Kerria lacca (plate 14.o) Piotr Naskrecki, Bud bug (plate 17.e) Beatriz Moisset: Sweat bee (plate 26.h) Lindsay Condon: Mole cricket (plate 28.l) Ashish Tiple: Common hooktail (plate 29.d) Ashish Tiple: Common clubtail (plate 29.e) Aleksandr: Lacewing larva (plate 34.c) Jeff Holman: Flea (plate 35.j) Kosta Mumcuoglu: Louse (plate 35.m) Erturac: Flea (plate 35.n) Cover: Amyciaea forticeps preying on Oecophylla smargdina, with a kleptoparasitic Phorid fly sharing in the meal. -
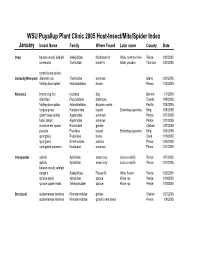
Host Insect List 2005
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

Work History Teacher's Assistant, Animal Behavior, Brown University
BILLY A. KRIMMEL Academic Training Sc.B. Brown University, 2008 (Human Biology); Honors in Biology Current Position Ph.D. Candidate in Ecology at UC Davis (Jay Rosenheim’s laboratory), 2009-; dissertation title: Plant traits and plant-herbivore-omnivore interactions Work History Teacher’s Assistant, Animal Behavior, Brown University, 2006, 2007 Teacher’s Assistant, Behavioral Ecology, Brown University, 2008 Instructor, All Kids Are Scientists (AKA Science), Portland OR, 2008-2009 Teacher’s Assistant, Introduction to Ecology and Evolution, UC Davis, 2010, 2011 Guest Instructor, Freshman Entomology Seminar, UC Davis, 2011 Guest Lecturer, California Wildflowers, American River College, 2014 Honors and Awards Royce Society Fellow, Brown University, 2006-2008 Senior Prize in Biology, Brown University, 2008 NSF Graduate Research Fellowship (GRF), 2011- 2014 Jastro Shields Fellowship, UC Davis, 2011 Robert van den Bosch Scholarship, University of California, 2012, 2013, 2014 UC Directors' Scholarship, UC Davis, 2013, 2014 Mildred Mathias Scholarship, University of California, 2013 Finalist, Lots of Opportunity Competition, Louisville, KY, 2014 UC Davis Business Development Fellow, 2014-2015 Publications Krimmel BA & Wheeler AG (in review) Hostplant stickiness disrupts novel ant-mealybug association. Arthropod-Plant Interactions Wheeler AG & Krimmel BA (in press) Mirid (Heteroptera) specialists of sticky plants: Adaptations, Interactions, and Ecological Implications. Annual Review of Entomology. Publication date: January 2015 Krimmel BA & Pearse IS (2014) Generalist and sticky plant specialist predators effectively suppress herbivores on a sticky plant. Arthropod-Plant Interactions 8: 403-410 Krimmel BA (2014) Why plant trichomes might be better than we think for predatory insects. Pest Management Science 70(11): 1666-1667 Wheeler AG & Krimmel BA (2014) Kleidocerys obovatus Van Duzee (Hemiptera: Lygaeidae: Ischnorhynchinae): New Distribution Records and Habits of an Apparent Seed Specialist on Cypress, Hesperocyparis spp. -

Recerca I Territori V12 B (002)(1).Pdf
Butterfly and moths in l’Empordà and their response to global change Recerca i territori Volume 12 NUMBER 12 / SEPTEMBER 2020 Edition Graphic design Càtedra d’Ecosistemes Litorals Mediterranis Mostra Comunicació Parc Natural del Montgrí, les Illes Medes i el Baix Ter Museu de la Mediterrània Printing Gràfiques Agustí Coordinadors of the volume Constantí Stefanescu, Tristan Lafranchis ISSN: 2013-5939 Dipòsit legal: GI 896-2020 “Recerca i Territori” Collection Coordinator Printed on recycled paper Cyclus print Xavier Quintana With the support of: Summary Foreword ......................................................................................................................................................................................................... 7 Xavier Quintana Butterflies of the Montgrí-Baix Ter region ................................................................................................................. 11 Tristan Lafranchis Moths of the Montgrí-Baix Ter region ............................................................................................................................31 Tristan Lafranchis The dispersion of Lepidoptera in the Montgrí-Baix Ter region ...........................................................51 Tristan Lafranchis Three decades of butterfly monitoring at El Cortalet ...................................................................................69 (Aiguamolls de l’Empordà Natural Park) Constantí Stefanescu Effects of abandonment and restoration in Mediterranean meadows .......................................87 -

Lepidoptera, Lycaenida
Boletín de la SAE Nº 19 (2012): 43-74 ISSN: 1578-1666 ISSN: 2254-8777 Consideraciones sobre la diversidad cromática de la familia Zygaenidae Latreille, 1809 (Insecta: Lepidoptera) Fidel FERNÁNDEZ-RUBIO1 1 Paseo de la Castellana, 138, 3º-28046 MADRID [email protected] RESUMEN: El trabajo muestra una breve descripción actualizada sobre la taxonomía y filogenia de la familia Zygaenidae, señalando la capacidad de sus especies de sintetizar cianoglucósidos y destacando la transcendencia de este hecho en la aparición de colores aposemáticos, en todos los géneros de Zygaeninae, donde sus especies forman un mimetismo de Müller, con los consecuentes resultados defensivos frente a los depredadores. Se destaca la influencia de la altitud y temperatura ambiental en la intensidad cromática de las formas locales de sus especies. Se señala la presencia de esta coloración defensiva en las especies del único género Paleártico de Chacosiinae (Aglaope), a diferencia de la coloración críptica, de camuflaje, en todas las especies Paleárticas de Procridinae, donde la formación de cianoglucósidos es muy baja o no está comprobada. Se acompañan varios anexos: una lista revisada de todos los géneros, subgéneros y especies de Zyganoidea que colonizan la Península Ibérica (anexo 1), la etimología de los nombre de las especies citadas (anexo 2) y un glosario de los términos poco usuales (anexo 3). Se muestra una abundante iconografía de las especies y circunstancias citadas. PALABRAS CLAVE: Zygaenidae, cianoglucósidos, coloración aposemática, mimetismo de Müller. Considerations on the chromatic range of the family Zygaenidae Latreille, 1809 (Insecta: Lepidoptera) ABSTRACT: The taxonomy and phylogeny of Zygaenidae is outlined, indicating the capacity of its species to synthesize cyanoglucosides, emphasizing its transcendence in the appearance of aposematic colours in all the species of Zygaeninae, where their species form mimicry of Müller, with the consequent defensive results front to the predators. -

Do Pesticide Residues Have Enduring Negative Effect on Macroinvertebrates and Vertebrates in Fallow Rice Paddies?
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.06.451252; this version posted July 6, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Do pesticide residues have enduring negative effect on macroinvertebrates and vertebrates in fallow rice paddies? Jheng-Sin Song, Chi-Chien Kuo* Department of Life Science, National Taiwan Normal University, Taipei, Taiwan *Corresponding author: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.07.06.451252; this version posted July 6, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Abstract 2 Rice is one of the most important staple food in the world, with irrigated rice paddies 3 largely converted from natural wetlands. The effectiveness of rice fields in help preserve 4 species depends partially on management practices, including the usage of pesticides. 5 However, related studies have focused predominately on the cultivation period, leaving the 6 effects of soil pesticide residues on aquatic invertebrates during the fallow periods little 7 explored; other animals, such as waterbirds, also rely on aquatic invertebrates in flooded 8 fallow fields for their survival. We therefore investigated vertebrates and macroinvertebrates 9 (terrestrial and aquatic) on rice stands and in flooded water during cultivation and fallow 10 periods in organic and conventional rice fields in Taiwan. -

Insect Classification Standards 2020
RECOMMENDED INSECT CLASSIFICATION FOR UGA ENTOMOLOGY CLASSES (2020) In an effort to standardize the hexapod classification systems being taught to our students by our faculty in multiple courses across three UGA campuses, I recommend that the Entomology Department adopts the basic system presented in the following textbook: Triplehorn, C.A. and N.F. Johnson. 2005. Borror and DeLong’s Introduction to the Study of Insects. 7th ed. Thomson Brooks/Cole, Belmont CA, 864 pp. This book was chosen for a variety of reasons. It is widely used in the U.S. as the textbook for Insect Taxonomy classes, including our class at UGA. It focuses on North American taxa. The authors were cautious, presenting changes only after they have been widely accepted by the taxonomic community. Below is an annotated summary of the T&J (2005) classification. Some of the more familiar taxa above the ordinal level are given in caps. Some of the more important and familiar suborders and families are indented and listed beneath each order. Note that this is neither an exhaustive nor representative list of suborders and families. It was provided simply to clarify which taxa are impacted by some of more important classification changes. Please consult T&J (2005) for information about taxa that are not listed below. Unfortunately, T&J (2005) is now badly outdated with respect to some significant classification changes. Therefore, in the classification standard provided below, some well corroborated and broadly accepted updates have been made to their classification scheme. Feel free to contact me if you have any questions about this classification. -

The Pyrenees
The Pyrenees A Greentours Holiday for the Alpine Garden Society 10th to 23rd June 2011 Led by Paul Cardy Trip Report and Systematic Lists by Paul Cardy Day 1 Friday 10 th June Arrival and Transfer to Formigueres Having driven from the south western Alps and reached Carcassonne the previous evening, I continued to Toulouse to meet the group at the airport. I was unexpectedly delayed by French customs who stopped me at the toll booth entering the city. There followed a lengthy questioning, as I had to unpack the contents of my suspiciously empty Italian mini-bus and show them my two large boxes of books, suitcase full of clothes, picnic supplies, etc., to convince them my purpose was a botanical tour to the Pyrenees. Now a little late I arrived breathlessly at Toulouse airport and rushed to the gate to meet Margaret, and the New Zealand contingent of Chris, Monica, Archie and Lynsie, hurriedly explaining the delay. Anyway we were soon back on the motorway and heading south towards Foix. White Storks in a field on route was a surprise. We made a picnic stop at a functional aire where there were tables, and a selection of weedy plants. Black Kite soared overhead. Once past Foix and Ax-les- Thermes the scenery became ever more interesting as we wound our way up to a misty Col de Puymorens. There a short stop yielded Pulsatilla vernalis in fruit and Trumpet Gentians. Roadside cliffs had Rock Soapwort, Saxifraga paniculata , and Elder-flowered Orchids became numerous. Now in the Parc Naturel Régional des Pyrénées Catalanes, a fascinating route down into the valley took us through Saillagouse and Mont-Louis before heading up a minor road to the village of Formigueres, our base for the first three nights. -

LYGAEOIDEA La Superfamila Lygaeoidea (Hemiptera: Heteroptera: Pentatomomorpha) Es Una De Las Mayores Y Más Diver- Sas, Con Más De 4000 Especies, De Heteroptera
| 421 Resumen LYGAEOIDEA La superfamila Lygaeoidea (Hemiptera: Heteroptera: Pentatomomorpha) es una de las mayores y más diver- sas, con más de 4000 especies, de Heteroptera. Los hábitats de las especies del grupo son variados, hay grupos arbóreos, geófilos y laminófilos. La mayoría se alimentan de semillas maduras, aunque las Blissidae y algunas Lygaeidae son succionadoras de savia, los Geocoridae son principalmente depredadoras y las Cle- radini (Rhyparochromidae) se alimentan de sangre de vertebrados. Las ninfas viven en los mismos hábitats que los adultos y se alimentan generalmente de las mismas plantas. Actualmente en los Lygaeoidea se reconocen 15 familias, de las cuales 12 han sido registradas de la región Neotropical y 11 de la Argentina: Berytidae, Blissidae, Colobathristidae, Cymidae, Geocoridae, Lygaeidae, Ninidae, Oxycarenidae, Pachygronthidae, Piesmatidae y Rhyparochromidae. Se presenta una breve historia taxonómica, aspectos filogenéticos y de la clasificación actual de la superfamilia, bibliografía de referencia y una clave para la identificación de las familias de la Argentina. Para cada familia se presenta una diagnosis, principales trabajos, aspectos de la bio- logía y la diversidad a nivel mundial y en la Argentina, así como claves para la determinación de los géneros presentes en el pais. Además, se reseña la importancia agroeconómica del grupo. Se adjunta un listado de las 154 especies citadas de Argentina. Pablo Matías DELLAPÉ Abstract The superfamily Lygaeoidea (Hemiptera: Heteroptera: División Entomología, Museo de La Plata, Paseo del Pentatomomorpha) is one of the most diverse within the Bosque, 1900 La Plata, Argentina. Heteroptera, with more than 4000 species described. [email protected] The Lygaeoid habitats are diverse; there are arboreal, geophile and laminophile species. -

Ebonyi State Government Nigeria Erosion and Watershed
SFG1692 V12 Environmental and Social Management Plan (ESMP) for Iyiokwu International Market Flood Site (Second Draft Report) EBONYI STATE GOVERNMENT NIGERIA Public Disclosure Authorized EROSION AND WATERSHED MANAGEMENT PROJECT (NEWMAP) Public Disclosure Authorized ENVIRONMENTAL AND SOCIAL MANAGEMENT PLAN (ESMP) FOR IYIOKWU- INTERNATIONAL MARKET FLOOD SITE IN ABAKALIKI Public Disclosure Authorized Public Disclosure Authorized FINAL REPORT DECEMBER, 2016 Page | i Environmental and Social Management Plan (ESMP) for Iyiokwu International Market Flood Site (Final Report) Table of Content Table of Content .............................................................................................................................................. ii LIST OF FIGURES ............................................................................................................................................. iv LIST OF TABLES ................................................................................................................................................ v LIST OF ACRONYMS ........................................................................................................................................ vi EXECUTIVE SUMMARY .................................................................................................................................. vii CHAPTER ONE .................................................................................................................................................. 1 1.0 INTRODUCTION