Conservation Biology, Husbandry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

50 CFR Ch. I (10–1–20 Edition) § 16.14
§ 15.41 50 CFR Ch. I (10–1–20 Edition) Species Common name Serinus canaria ............................................................. Common Canary. 1 Note: Permits are still required for this species under part 17 of this chapter. (b) Non-captive-bred species. The list 16.14 Importation of live or dead amphib- in this paragraph includes species of ians or their eggs. non-captive-bred exotic birds and coun- 16.15 Importation of live reptiles or their tries for which importation into the eggs. United States is not prohibited by sec- Subpart C—Permits tion 15.11. The species are grouped tax- onomically by order, and may only be 16.22 Injurious wildlife permits. imported from the approved country, except as provided under a permit Subpart D—Additional Exemptions issued pursuant to subpart C of this 16.32 Importation by Federal agencies. part. 16.33 Importation of natural-history speci- [59 FR 62262, Dec. 2, 1994, as amended at 61 mens. FR 2093, Jan. 24, 1996; 82 FR 16540, Apr. 5, AUTHORITY: 18 U.S.C. 42. 2017] SOURCE: 39 FR 1169, Jan. 4, 1974, unless oth- erwise noted. Subpart E—Qualifying Facilities Breeding Exotic Birds in Captivity Subpart A—Introduction § 15.41 Criteria for including facilities as qualifying for imports. [Re- § 16.1 Purpose of regulations. served] The regulations contained in this part implement the Lacey Act (18 § 15.42 List of foreign qualifying breed- U.S.C. 42). ing facilities. [Reserved] § 16.2 Scope of regulations. Subpart F—List of Prohibited Spe- The provisions of this part are in ad- cies Not Listed in the Appen- dition to, and are not in lieu of, other dices to the Convention regulations of this subchapter B which may require a permit or prescribe addi- § 15.51 Criteria for including species tional restrictions or conditions for the and countries in the prohibited list. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -
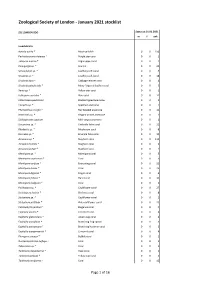
Jan 2021 ZSL Stocklist.Pdf (699.26
Zoological Society of London - January 2021 stocklist ZSL LONDON ZOO Status at 01.01.2021 m f unk Invertebrata Aurelia aurita * Moon jellyfish 0 0 150 Pachyclavularia violacea * Purple star coral 0 0 1 Tubipora musica * Organ-pipe coral 0 0 2 Pinnigorgia sp. * Sea fan 0 0 20 Sarcophyton sp. * Leathery soft coral 0 0 5 Sinularia sp. * Leathery soft coral 0 0 18 Sinularia dura * Cabbage leather coral 0 0 4 Sinularia polydactyla * Many-fingered leather coral 0 0 3 Xenia sp. * Yellow star coral 0 0 1 Heliopora coerulea * Blue coral 0 0 12 Entacmaea quadricolor Bladdertipped anemone 0 0 1 Epicystis sp. * Speckled anemone 0 0 1 Phymanthus crucifer * Red beaded anemone 0 0 11 Heteractis sp. * Elegant armed anemone 0 0 1 Stichodactyla tapetum Mini carpet anemone 0 0 1 Discosoma sp. * Umbrella false coral 0 0 21 Rhodactis sp. * Mushroom coral 0 0 8 Ricordea sp. * Emerald false coral 0 0 19 Acropora sp. * Staghorn coral 0 0 115 Acropora humilis * Staghorn coral 0 0 1 Acropora yongei * Staghorn coral 0 0 2 Montipora sp. * Montipora coral 0 0 5 Montipora capricornis * Coral 0 0 5 Montipora confusa * Encrusting coral 0 0 22 Montipora danae * Coral 0 0 23 Montipora digitata * Finger coral 0 0 6 Montipora foliosa * Hard coral 0 0 10 Montipora hodgsoni * Coral 0 0 2 Pocillopora sp. * Cauliflower coral 0 0 27 Seriatopora hystrix * Bird nest coral 0 0 8 Stylophora sp. * Cauliflower coral 0 0 1 Stylophora pistillata * Pink cauliflower coral 0 0 23 Catalaphyllia jardinei * Elegance coral 0 0 4 Euphyllia ancora * Crescent coral 0 0 4 Euphyllia glabrescens * Joker's cap coral 0 0 2 Euphyllia paradivisa * Branching frog spawn 0 0 3 Euphyllia paraancora * Branching hammer coral 0 0 3 Euphyllia yaeyamaensis * Crescent coral 0 0 4 Plerogyra sinuosa * Bubble coral 0 0 1 Duncanopsammia axifuga + Coral 0 0 2 Tubastraea sp. -

The Classification of Lower Organisms
The Classification of Lower Organisms Ernst Hkinrich Haickei, in 1874 From Rolschc (1906). By permission of Macrae Smith Company. C f3 The Classification of LOWER ORGANISMS By HERBERT FAULKNER COPELAND \ PACIFIC ^.,^,kfi^..^ BOOKS PALO ALTO, CALIFORNIA Copyright 1956 by Herbert F. Copeland Library of Congress Catalog Card Number 56-7944 Published by PACIFIC BOOKS Palo Alto, California Printed and bound in the United States of America CONTENTS Chapter Page I. Introduction 1 II. An Essay on Nomenclature 6 III. Kingdom Mychota 12 Phylum Archezoa 17 Class 1. Schizophyta 18 Order 1. Schizosporea 18 Order 2. Actinomycetalea 24 Order 3. Caulobacterialea 25 Class 2. Myxoschizomycetes 27 Order 1. Myxobactralea 27 Order 2. Spirochaetalea 28 Class 3. Archiplastidea 29 Order 1. Rhodobacteria 31 Order 2. Sphaerotilalea 33 Order 3. Coccogonea 33 Order 4. Gloiophycea 33 IV. Kingdom Protoctista 37 V. Phylum Rhodophyta 40 Class 1. Bangialea 41 Order Bangiacea 41 Class 2. Heterocarpea 44 Order 1. Cryptospermea 47 Order 2. Sphaerococcoidea 47 Order 3. Gelidialea 49 Order 4. Furccllariea 50 Order 5. Coeloblastea 51 Order 6. Floridea 51 VI. Phylum Phaeophyta 53 Class 1. Heterokonta 55 Order 1. Ochromonadalea 57 Order 2. Silicoflagellata 61 Order 3. Vaucheriacea 63 Order 4. Choanoflagellata 67 Order 5. Hyphochytrialea 69 Class 2. Bacillariacea 69 Order 1. Disciformia 73 Order 2. Diatomea 74 Class 3. Oomycetes 76 Order 1. Saprolegnina 77 Order 2. Peronosporina 80 Order 3. Lagenidialea 81 Class 4. Melanophycea 82 Order 1 . Phaeozoosporea 86 Order 2. Sphacelarialea 86 Order 3. Dictyotea 86 Order 4. Sporochnoidea 87 V ly Chapter Page Orders. Cutlerialea 88 Order 6. -

EC) No 338/97 on the Protection of Species of Wild Fauna and Flora by Regulating Trade Therein
12.8.2010 EN Official Journal of the European Union L 212/1 II (Non-legislative acts) REGULATIONS COMMISSION REGULATION (EU) No 709/2010 of 22 July 2010 amending Council Regulation (EC) No 338/97 on the protection of species of wild fauna and flora by regulating trade therein THE EUROPEAN COMMISSION, (7) The species Ctenosaura bakeri, C. oedirhina, C. melanosterna, C. palearis, Agalychnis spp., Dynastes satanas, Operculicarya hyphaenoides, O. pachypus, Zygosicyos pubescens, Z. Having regard to the Treaty on the Functioning of the European tripartitus, Aniba rosaeodora (with annotation), Adenia Union, olaboensis, Cyphostemma elephantopus, C. montagnacii and Bulnesia sarmientoi (with annotation) have been included in Appendix II. Having regard to Council Regulation (EC) No 338/97 of 9 December 1996 on the protection of species of wild fauna 1 and flora by regulating trade therein ( ), and in particular (8) The Appendix II listing of Beccariophoenix madagascariensis Article 19(5) thereof, and Neodypsis decaryi was extended to include seeds from Madagascar. Whereas: (9) The following species have been deleted from Appendix III to the Convention at the request of Malaysia: Arbo (1) Regulation (EC) No 338/97 lists animal and plant species rophila campbelli, Arborophila charltonii, Caloperdix oculeus, in respect of which trade is restricted or controlled. Lophura erythrophthalma, Lophura ignita, Melanoperdix niger, Those lists incorporate the lists set out in the Appendices Polyplectron inopinatum, Rhizothera dulitensis, Rhizothera to the Convention on International Trade in Endangered longirostris and Rollulus rouloul, and the species Haliotis Species of Wild Fauna and Flora, hereinafter ‘the midae has been deleted from Appendix III to the Convention’. -

A Conservation Reassessment of the Critically Endangered, Lorestan Newt Neurergus Kaiseri (Schmidt 1952) in Iran
Copyright: © This is an open-access article distributed under the terms of the Creative Commons Attribution–Non Commercial –No Derivs 3.0 Unported License, which permits conditional use for non-commercial and education purposes, provided the Amphibian and Reptile Conservation 9(1): 16- original author and source are credited, and prohibits the deposition of material from www.redlist -arc.org or affiliated websites 25. including www.redlist.arcme.org, including PDFs, images, or text, onto other websites without permission of the Amphibian and Reptile Conservation journal at www.redlist-arc.org A conservation reassessment of the Critically Endangered, Lorestan newt Neurergus kaiseri (Schmidt 1952) in Iran 1* 2 3 1 2 Asghar Mobaraki , Mohsen Amiri , Rahim Alvandi , Masoud Ebrahim Tehrani , Hossein Zarin Kia , Ali 2 4, 5 6 Khoshnamvand , Ali Bali Ehsan Forozanfar , and Robert K Browne 1: Department of Environment, Biodiversity and Wildlife Bureau, PO. Box 14155-7383, Tehran, Iran, 2: Department of Environment Provincial Office in Lorestan, Khoram Abad, Iran, 3: Department of Environment Provincial Office in Khuzestan, Ahvaz, Iran 4: Department of Environment, Habitats and Protected Areas Affairs Bureau, PO. Box 14155-7383, Tehran, Iran, 5: Faculty of Environment, Standard Square, Karaj, 6: Sustainability America, Sarteneja, Belize. Abstract The Lorestan newt (Neurergus kaiseri, Schmidt 1952) is an endemic salamander species to Iran, listed as “Critically Endangered” in the 2006 IUCN Red List due to population declines of 80%, over collection for the pet trade; area of occupancy less than 10 km2, fragmented populations, less than 1,000 adults, and continuing habitat degradation and loss. However, the Red List assessment was limited to surveys only around the type location of N. -

No Evidence for the 'Rate-Of-Living' Theory Across the Tetrapod Tree of Life
Received: 23 June 2019 | Revised: 30 December 2019 | Accepted: 7 January 2020 DOI: 10.1111/geb.13069 RESEARCH PAPER No evidence for the ‘rate-of-living’ theory across the tetrapod tree of life Gavin Stark1 | Daniel Pincheira-Donoso2 | Shai Meiri1,3 1School of Zoology, Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel Abstract 2School of Biological Sciences, Queen’s Aim: The ‘rate-of-living’ theory predicts that life expectancy is a negative function of University Belfast, Belfast, United Kingdom the rates at which organisms metabolize. According to this theory, factors that accel- 3The Steinhardt Museum of Natural History, Tel Aviv University, Tel Aviv, Israel erate metabolic rates, such as high body temperature and active foraging, lead to organismic ‘wear-out’. This process reduces life span through an accumulation of bio- Correspondence Gavin Stark, School of Zoology, Faculty of chemical errors and the build-up of toxic metabolic by-products. Although the rate- Life Sciences, Tel Aviv University, Tel Aviv, of-living theory is a keystone underlying our understanding of life-history trade-offs, 6997801, Israel. Email: [email protected] its validity has been recently questioned. The rate-of-living theory has never been tested on a global scale in a phylogenetic framework, or across both endotherms and Editor: Richard Field ectotherms. Here, we test several of its fundamental predictions across the tetrapod tree of life. Location: Global. Time period: Present. Major taxa studied: Land vertebrates. Methods: Using a dataset spanning the life span data of 4,100 land vertebrate spe- cies (2,214 endotherms, 1,886 ectotherms), we performed the most comprehensive test to date of the fundamental predictions underlying the rate-of-living theory. -

Notes on the Distribution and Abundance of the Endangered Kaiser ’S Mountain Newt , Neurergus Kaiseri (C Audata : Salamandridae ), in Southwestern Iran
Herpetological Conservation and Biology 8(3):724−731. HSuebrpmeittotelodg: i1c aJlu Clyo 2n0se1r2v;a Aticocne apntedd B: 9io Jlouglyy 2013; Published: 31 December 2013. Notes oN the DistributioN aND abuNDaNce of the eNDaNgereD Kaiser ’s MouNtaiN Newt , Neurergus kaiseri (c auData : salaMaNDriDae ), iN southwesterN iraN Mozafar sharifi 1* , h osseiN farasat 1, h osseiN BaraNi -B eiraNv 2, soMaye vaissi 1, and ehsaN foroozaNfar 3 1Razi University Center for Environmental Studies, Department of Biology, Faculty of Sciences, Kermanshah 67149, Iran 2Department of Biology, Faculty of Sciences, Ferdowsi University of Mashhad, Mashhad, Iran 3Faculty of Environment, Ostandari Square, Karaj, Iran *Corresponding author, e-mail: [email protected] abstract. —the endangered Kaiser’s Mountain Newt, Neurergus kaiseri , is a species endemic to the southern Zagros range in iran. until now, N. kaiseri had been reported from only five localities. the present study describes eight new localities that increase the species’ known range from 212 km 2 (minimum convex polygon) to 789 km 2 at elevations between 930−1395 meters. the localities are generally dispersed; nearest neighbor distances among the 13 localities average 4.61 km (range, 0.93−12.39 km). the streams are separated from one another by steep and rocky terrain with vegetation cover of mature open oak woodland in the west and sparse scrubland or thin oak-pistachio woodland in the central area and east, thus potentially isolating many of the populations from each other. we surveyed 12 of the 13 localities with newts 1−2 times and counted a total of 1,277 adults, post-metamorphic sub-adults, and larvae (mean per stream, 106; range, 2−650) in a total of 4.23 km of stream reaches. -

Feeding in Amphibians: Evolutionary Transformations and Phenotypic Diversity As Drivers of Feeding System Diversity
Chapter 12 Feeding in Amphibians: Evolutionary Transformations and Phenotypic Diversity as Drivers of Feeding System Diversity Anthony Herrel, James C. O’Reilly, Anne-Claire Fabre, Carla Bardua, Aurélien Lowie, Renaud Boistel and Stanislav N. Gorb Abstract Amphibians are different from most other tetrapods because they have a biphasic life cycle, with larval forms showing a dramatically different cranial anatomy and feeding strategy compared to adults. Amphibians with their exceptional diversity in habitats, lifestyles and reproductive modes are also excellent models for studying the evolutionary divergence in feeding systems. In the present chapter, we review the literature on amphibian feeding anatomy and function published since 2000. We also present some novel unpublished data on caecilian feeding biome- chanics. This review shows that over the past two decades important new insights in our understanding of amphibian feeding anatomy and function have been made possible, thanks to a better understanding of the phylogenetic relationships between taxa, analyses of development and the use of biomechanical modelling. In terms of functional analyses, important advances involve the temperature-dependent nature of tongue projection mechanisms and the plasticity exhibited by animals when switch- A. Herrel (B) Département Adaptations du Vivant, Muséum national d’Histoire naturelle, UMR 7179 C.N.R.S/M.N.H.N, 55 rue Buffon, 75005, Paris Cedex 05, France e-mail: [email protected] J. C. O’Reilly Department of Biomedical Sciences, Ohio University, Cleveland Campus, Cleveland, Ohio 334C, USA A.-C. Fabre · C. Bardua Life Sciences Department, The Natural History Museum, Cromwell Road, London SW7 5BD, UK A. Lowie Department of Biology Evolutionary, Morphology of Vertebrates, Ghent University, K.L. -

Salamander Species Listed As Injurious Wildlife Under 50 CFR 16.14 Due to Risk of Salamander Chytrid Fungus Effective January 28, 2016
Salamander Species Listed as Injurious Wildlife Under 50 CFR 16.14 Due to Risk of Salamander Chytrid Fungus Effective January 28, 2016 Effective January 28, 2016, both importation into the United States and interstate transportation between States, the District of Columbia, the Commonwealth of Puerto Rico, or any territory or possession of the United States of any live or dead specimen, including parts, of these 20 genera of salamanders are prohibited, except by permit for zoological, educational, medical, or scientific purposes (in accordance with permit conditions) or by Federal agencies without a permit solely for their own use. This action is necessary to protect the interests of wildlife and wildlife resources from the introduction, establishment, and spread of the chytrid fungus Batrachochytrium salamandrivorans into ecosystems of the United States. The listing includes all species in these 20 genera: Chioglossa, Cynops, Euproctus, Hydromantes, Hynobius, Ichthyosaura, Lissotriton, Neurergus, Notophthalmus, Onychodactylus, Paramesotriton, Plethodon, Pleurodeles, Salamandra, Salamandrella, Salamandrina, Siren, Taricha, Triturus, and Tylototriton The species are: (1) Chioglossa lusitanica (golden striped salamander). (2) Cynops chenggongensis (Chenggong fire-bellied newt). (3) Cynops cyanurus (blue-tailed fire-bellied newt). (4) Cynops ensicauda (sword-tailed newt). (5) Cynops fudingensis (Fuding fire-bellied newt). (6) Cynops glaucus (bluish grey newt, Huilan Rongyuan). (7) Cynops orientalis (Oriental fire belly newt, Oriental fire-bellied newt). (8) Cynops orphicus (no common name). (9) Cynops pyrrhogaster (Japanese newt, Japanese fire-bellied newt). (10) Cynops wolterstorffi (Kunming Lake newt). (11) Euproctus montanus (Corsican brook salamander). (12) Euproctus platycephalus (Sardinian brook salamander). (13) Hydromantes ambrosii (Ambrosi salamander). (14) Hydromantes brunus (limestone salamander). (15) Hydromantes flavus (Mount Albo cave salamander). -

Thesis Style Document
The environmental toxicology of zinc oxide nanoparticles to the oligochaete Lumbriculus variegatus Shona Aisling O’Rourke Submitted for the degree of Doctor of Philosophy Heriot-Watt University School of Life Sciences January 2013 The copyright in this thesis is owned by the author. Any quotation from the thesis or use of any of the information contained in it must acknowledge this thesis as the source of the quotation or information. Abstract This thesis investigated the potential toxicity of zinc oxide nanoparticles (NPs) and bulk particles (both with and without organic matter (HA)) to the Californian Blackworm, Lumbriculus variegatus. The NPs and bulk particles in this thesis were characterised 133 using numerous techniques. ZnO NPs were found to be 91 ( 64) nm (median 322 (interquartile range)) and ZnO bulk particles were found to be 237 ( 165) nm (median (interquartile range)) by TEM. In the acute behavioural study (96 hour), ZnO NPs had a dose-dependent toxic effect on the behaviour of the worms up to 10mg/L whereas the bulk had no significant effect. This result, however, was mitigated by the addition of 5mg/L HA in the NP study whereas a similar addition enhanced the toxicity of the bulk particles at 5mg/L ZnO. In the chronic study (28 days), ZnO NPs and bulk particles were found to have a dose-dependent significant effect on the behaviour of the worms after 28 days, with NPs causing a significantly greater negative response than bulk particles at 12.5, 25 and 50mg/L ZnO. HA had no effect on the toxicity of either particle type in the chronic study. -

Amphibia, Urodela)
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/235246853 A new ergotaxonomy of the family Salamandridae Goldfuss, 1820 (Amphibia, Urodela) Article in Alytes · March 2009 CITATIONS READS 133 1,636 2 authors: Alain Dubois Jean Raffaëlli Muséum National d'Histoire Naturelle French Urodela Group 307 PUBLICATIONS 6,658 CITATIONS 14 PUBLICATIONS 293 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Taxonomy and nomenclature of amphibians View project Observatory on Availability in Zoological Nomenclature View project All content following this page was uploaded by Alain Dubois on 28 May 2014. The user has requested enhancement of the downloaded file. March 2009 Volume 26, No 1-4 Alytes, 2009, 26 (1-4): 1-85. A new ergotaxonomy of the family Salamandridae Goldfuss, 1820 (Amphibia, Urodela) Alain Dubois* & Jean Raffaëlli** * Reptiles & Amphibiens, UMR 5202 CNRS OSEB, Département Systématique & Evolution, Muséum national d’Histoire naturelle, CP 30, 25 rue Cuvier, 75005 Paris, France <[email protected]> ** Penclen, 56420 Plumelec, France <jean.raff[email protected]> Several recent studies, particularly dealing with molecular phylogeny, have improved our knowledge of the relationships within the salamander family SALAMANDRIDAE. However, some only of these findings have resulted in formal taxonomic changes. In order to homogenize this taxonomy, we hereby recognize several new taxa at various ranks from subfamily to subspecies,