Download the PDF Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LARVAL FORMS in ECHINODERMATA in Echinoderms
LARVAL FORMS IN ECHINODERMATA In echinoderms eggs and sperms are released in water and fertilization takes place in water forming zygote. Echinoderms are deuterostomes and hence cleavage is radial, holoblastic and indeterminate. The larvae hatch in water and feed and grow through successive larval stages to become adults. The larvae of echinoderms are bilaterally symmetrical but lose symmetry during metamorphosis. Different classes of echinoderms show structurally different larval stages and their comparisons can reveal their evolutionary ancestry. LARVAE OF ASTEROIDEA There are three larval stages in Asteroidea in the course of their development to adult stage. Early bipinnaria appears like hypothetical dipleurula. It has oval body without arms and ciliary bands for locomotion. It has well developed alimentary canal for feeding and grows to become bipinnaria. Bipinnaria larva possesses 5 pairs of ciliated arms which do not have any skeletal support inside. These arms are used for swimming in water while feeding on planktons. Preoral and postoral ciliary bands are also present. This larva resembles auricularia larva of Holothuroidea in general appearance. Brachiolaria larva is formed after 6-7 weeks of life and growth of bipinnaria. This larva is sedentary and remains attached to a hard substratum for which it possesses three brachiolarian arms having adhesive discs at the tip. Ciliated arms get reduced and become thin and functionless, while mouth, anus and gut are well developed. It has axocoel, hydocoel and somatocoel that later on give rise to water vascular system Development of starfish takes place inside the sedentary brachiolaria which ruptures and releases tiny starfish into water. LARVAE OF HOLOTHUROIDEA Class Holothuroidea demonstrate two larval stages, namely, auricularia and doliolaria larvae. -

A New Record of the Persian Brook Salamander, Paradactylodon Persicus (Eiselt & Steiner, 1970) (Amphibia: Caudata: Hynobiidae) in Northern Iran
Bonn zoological Bulletin Volume 60 Issue 1 pp. 63–65 Bonn, May 2011 A new record of the Persian Brook Salamander, Paradactylodon persicus (Eiselt & Steiner, 1970) (Amphibia: Caudata: Hynobiidae) in northern Iran Faraham Ahmadzadeh1, 2*, Fatemeh Khanjani3, Aref Shadkam4 & Wolfgang Böhme2 1Department of Biodiversity and Ecosystem Management, Environmental Sciences Research Institute, Shahid Beheshti University, Evin, Tehran, G. C., Iran 2Herpetology Section, Zoologisches Forschungsmuseum Alexander Koenig (ZFMK), Adenauerallee 160, D-53113 Bonn, Germany 3Department of Habitat and Biodiversity, Faculty of Environment and Energy, Science and Research Branch, Islamic Azad University, Ponak, Tehran, Iran 4Department of Environment, Rezvan Shahr, Province Gilan, Iran *Corresponding Email address: [email protected]. INTRODUCTION The Persian Brook Salamander, Paradactylodon persicus 90 mm; tail length 120 mm; head large, 20 mm in length; (Eiselt & Steiner, 1970) is an endemic and poorly known vomerine teeth in two arch-shaped rows; snout rounded; species of northern Iran (Baloutch & Kami 1995; Kami fore and hind limbs with four digits; tail flattened later- 1999). It was originally described as Batrachuperus per- ally, with round-tapered end; dorsal head and body, as well sicus by Eiselt & Steiner (1970), but has been transferred as upper surface of tail brownish with yellow spots and to the genus Paradactylodon based on genetic studies by marblings; belly cream without pattern (Fig. 2a–b). Zhang et al. (2006). This species has been reported from two localities only: Weyser, southeast of Chalus, in Paradactylodon persicus inhabits the mountainous Mazandaran Province (36º 30´ 35” N and 51º 26´ 38” E) streams and brooks, with cool, fast-flowing water and Delmadeh village, southeast of Khalkhal, in Ardabil (Baloutch & Kami 1995; Kami 1999; Ahmadzadeh & Ka- Province (37º 22´ 34” N and 48º 47´ 35” E) (Kami 2004; mi 2009). -

Amphibiaweb's Illustrated Amphibians of the Earth
AmphibiaWeb's Illustrated Amphibians of the Earth Created and Illustrated by the 2020-2021 AmphibiaWeb URAP Team: Alice Drozd, Arjun Mehta, Ash Reining, Kira Wiesinger, and Ann T. Chang This introduction to amphibians was written by University of California, Berkeley AmphibiaWeb Undergraduate Research Apprentices for people who love amphibians. Thank you to the many AmphibiaWeb apprentices over the last 21 years for their efforts. Edited by members of the AmphibiaWeb Steering Committee CC BY-NC-SA 2 Dedicated in loving memory of David B. Wake Founding Director of AmphibiaWeb (8 June 1936 - 29 April 2021) Dave Wake was a dedicated amphibian biologist who mentored and educated countless people. With the launch of AmphibiaWeb in 2000, Dave sought to bring the conservation science and basic fact-based biology of all amphibians to a single place where everyone could access the information freely. Until his last day, David remained a tirelessly dedicated scientist and ally of the amphibians of the world. 3 Table of Contents What are Amphibians? Their Characteristics ...................................................................................... 7 Orders of Amphibians.................................................................................... 7 Where are Amphibians? Where are Amphibians? ............................................................................... 9 What are Bioregions? ..................................................................................10 Conservation of Amphibians Why Save Amphibians? ............................................................................. -

50 CFR Ch. I (10–1–20 Edition) § 16.14
§ 15.41 50 CFR Ch. I (10–1–20 Edition) Species Common name Serinus canaria ............................................................. Common Canary. 1 Note: Permits are still required for this species under part 17 of this chapter. (b) Non-captive-bred species. The list 16.14 Importation of live or dead amphib- in this paragraph includes species of ians or their eggs. non-captive-bred exotic birds and coun- 16.15 Importation of live reptiles or their tries for which importation into the eggs. United States is not prohibited by sec- Subpart C—Permits tion 15.11. The species are grouped tax- onomically by order, and may only be 16.22 Injurious wildlife permits. imported from the approved country, except as provided under a permit Subpart D—Additional Exemptions issued pursuant to subpart C of this 16.32 Importation by Federal agencies. part. 16.33 Importation of natural-history speci- [59 FR 62262, Dec. 2, 1994, as amended at 61 mens. FR 2093, Jan. 24, 1996; 82 FR 16540, Apr. 5, AUTHORITY: 18 U.S.C. 42. 2017] SOURCE: 39 FR 1169, Jan. 4, 1974, unless oth- erwise noted. Subpart E—Qualifying Facilities Breeding Exotic Birds in Captivity Subpart A—Introduction § 15.41 Criteria for including facilities as qualifying for imports. [Re- § 16.1 Purpose of regulations. served] The regulations contained in this part implement the Lacey Act (18 § 15.42 List of foreign qualifying breed- U.S.C. 42). ing facilities. [Reserved] § 16.2 Scope of regulations. Subpart F—List of Prohibited Spe- The provisions of this part are in ad- cies Not Listed in the Appen- dition to, and are not in lieu of, other dices to the Convention regulations of this subchapter B which may require a permit or prescribe addi- § 15.51 Criteria for including species tional restrictions or conditions for the and countries in the prohibited list. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -
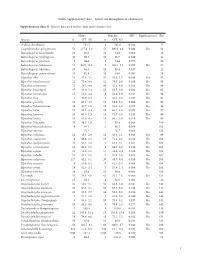
I Online Supplementary Data – Sexual Size Dimorphism in Salamanders
Online Supplementary data – Sexual size dimorphism in salamanders Supplementary data S1. Species data used in this study and references list. Males Females SSD Significant test Ref Species n SVL±SD n SVL±SD Andrias davidianus 2 532.5 8 383.0 -0.280 12 Cryptobranchus alleganiensis 53 277.4±5.2 52 300.9±3.4 0.084 Yes 61 Batrachuperus karlschmidti 10 80.0 10 84.8 0.060 26 Batrachuperus londongensis 20 98.6 10 96.7 -0.019 12 Batrachuperus pinchonii 5 69.6 5 74.6 0.070 26 Batrachuperus taibaiensis 11 92.9±12.1 9 102.1±7.1 0.099 Yes 27 Batrachuperus tibetanus 10 94.5 10 92.8 -0.017 12 Batrachuperus yenyuadensis 10 82.8 10 74.8 -0.096 26 Hynobius abei 24 57.8±2.1 34 55.0±1.2 -0.048 Yes 92 Hynobius amakusaensis 22 75.4±4.8 12 76.5±3.6 0.014 No 93 Hynobius arisanensis 72 54.3±4.8 40 55.2±4.8 0.016 No 94 Hynobius boulengeri 37 83.0±5.4 15 91.5±3.8 0.102 Yes 95 Hynobius formosanus 15 53.0±4.4 8 52.4±3.9 -0.011 No 94 Hynobius fuca 4 50.9±2.8 3 52.8±2.0 0.037 No 94 Hynobius glacialis 12 63.1±4.7 11 58.9±5.2 -0.066 No 94 Hynobius hidamontanus 39 47.7±1.0 15 51.3±1.2 0.075 Yes 96 Hynobius katoi 12 58.4±3.3 10 62.7±1.6 0.073 Yes 97 Hynobius kimurae 20 63.0±1.5 15 72.7±2.0 0.153 Yes 98 Hynobius leechii 70 61.6±4.5 18 66.5±5.9 0.079 Yes 99 Hynobius lichenatus 37 58.5±1.9 2 53.8 -0.080 100 Hynobius maoershanensis 4 86.1 2 80.1 -0.069 101 Hynobius naevius 72.1 76.7 0.063 102 Hynobius nebulosus 14 48.3±2.9 12 50.4±2.1 0.043 Yes 96 Hynobius osumiensis 9 68.4±3.1 15 70.2±3.0 0.026 No 103 Hynobius quelpaertensis 41 52.5±3.8 4 61.3±4.1 0.167 Yes 104 Hynobius -

EC) No 338/97 on the Protection of Species of Wild Fauna and Flora by Regulating Trade Therein
12.8.2010 EN Official Journal of the European Union L 212/1 II (Non-legislative acts) REGULATIONS COMMISSION REGULATION (EU) No 709/2010 of 22 July 2010 amending Council Regulation (EC) No 338/97 on the protection of species of wild fauna and flora by regulating trade therein THE EUROPEAN COMMISSION, (7) The species Ctenosaura bakeri, C. oedirhina, C. melanosterna, C. palearis, Agalychnis spp., Dynastes satanas, Operculicarya hyphaenoides, O. pachypus, Zygosicyos pubescens, Z. Having regard to the Treaty on the Functioning of the European tripartitus, Aniba rosaeodora (with annotation), Adenia Union, olaboensis, Cyphostemma elephantopus, C. montagnacii and Bulnesia sarmientoi (with annotation) have been included in Appendix II. Having regard to Council Regulation (EC) No 338/97 of 9 December 1996 on the protection of species of wild fauna 1 and flora by regulating trade therein ( ), and in particular (8) The Appendix II listing of Beccariophoenix madagascariensis Article 19(5) thereof, and Neodypsis decaryi was extended to include seeds from Madagascar. Whereas: (9) The following species have been deleted from Appendix III to the Convention at the request of Malaysia: Arbo (1) Regulation (EC) No 338/97 lists animal and plant species rophila campbelli, Arborophila charltonii, Caloperdix oculeus, in respect of which trade is restricted or controlled. Lophura erythrophthalma, Lophura ignita, Melanoperdix niger, Those lists incorporate the lists set out in the Appendices Polyplectron inopinatum, Rhizothera dulitensis, Rhizothera to the Convention on International Trade in Endangered longirostris and Rollulus rouloul, and the species Haliotis Species of Wild Fauna and Flora, hereinafter ‘the midae has been deleted from Appendix III to the Convention’. -

Bonn Zoological Bulletin Volume 60 Issue 1 Pp
1 © Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zoologicalbulletin.de; www.biologiezentrum.at Bonn zoological Bulletin Volume 60 Issue 1 pp. 63-65 Bonn, May 201 A new record of the Persian Brook Salamander, Paradactylodon persicus (Eiselt & Steiner, 1970) (Amphibia: Caudata: Hynobiidae) in northern Iran 1 2 3 4 2 - Faraham Ahmadzadeh \ Fatemeh Khanjani , Aref Shadkam & Wolfgang Bohme 'Department of Biodiversity and Ecosystem Management, Environmental Sciences Research Institute, Shahid Beheshti University, Evin, Tehran, G. C, Iran 2 Herpetology Section, Zoologisches Forschungsmuseum Alexander Koenig (ZFMK), Adenauerallee 160, D-53113 Bonn, Germany 3 Department of Habitat and Biodiversity, Faculty of Environment and Energy, Science and Research Branch, Islamic Azad University, Ponak, Tehran, Iran 4 Department of Environment, Rezvan Shahr, Province Gilan, Iran "Corresponding Email address: [email protected]. INTRODUCTION The Persian Brook Salamander, Paradactylodon persicus 90 mm; tail length 120 mm; head large, 20 mm in length; (Eiselt & Steiner, 1970) is an endemic and poorly known vomerine teeth in two arch-shaped rows; snout rounded; species of northern Iran (Baloutch & Kami 1995; Kami fore and hind limbs with four digits; tail flattened later- 1999). It was originally described as Batrachuperus per- ally, with round-tapered end; dorsal head and body, as well sicus by Eiselt & Steiner ( 1 970), but has been transferred as upper surface of tail brownish with yellow spots and to the genus Paradactylodon based on genetic studies by marblings; belly cream without pattern (Fig. 2a-b). Zhang et al. (2006). This species has been reported from two localities only: Weyser, southeast of Chalus, in Paradactylodon persicus inhabits the mountainous Mazandaran Province (36° 30' 35" N and 51° 26' 38" E) streams and brooks, with cool, fast-flowing water and Delmadeh village, southeast of Khalkhal, in Ardabil (Baloutch & Kami 1995; Kami 1999; Ahmadzadeh & Ka- Province (37° 22' 34" N and 48° 47' 35" E) (Kami 2004; mi 2009). -

A Conservation Reassessment of the Critically Endangered, Lorestan Newt Neurergus Kaiseri (Schmidt 1952) in Iran
Copyright: © This is an open-access article distributed under the terms of the Creative Commons Attribution–Non Commercial –No Derivs 3.0 Unported License, which permits conditional use for non-commercial and education purposes, provided the Amphibian and Reptile Conservation 9(1): 16- original author and source are credited, and prohibits the deposition of material from www.redlist -arc.org or affiliated websites 25. including www.redlist.arcme.org, including PDFs, images, or text, onto other websites without permission of the Amphibian and Reptile Conservation journal at www.redlist-arc.org A conservation reassessment of the Critically Endangered, Lorestan newt Neurergus kaiseri (Schmidt 1952) in Iran 1* 2 3 1 2 Asghar Mobaraki , Mohsen Amiri , Rahim Alvandi , Masoud Ebrahim Tehrani , Hossein Zarin Kia , Ali 2 4, 5 6 Khoshnamvand , Ali Bali Ehsan Forozanfar , and Robert K Browne 1: Department of Environment, Biodiversity and Wildlife Bureau, PO. Box 14155-7383, Tehran, Iran, 2: Department of Environment Provincial Office in Lorestan, Khoram Abad, Iran, 3: Department of Environment Provincial Office in Khuzestan, Ahvaz, Iran 4: Department of Environment, Habitats and Protected Areas Affairs Bureau, PO. Box 14155-7383, Tehran, Iran, 5: Faculty of Environment, Standard Square, Karaj, 6: Sustainability America, Sarteneja, Belize. Abstract The Lorestan newt (Neurergus kaiseri, Schmidt 1952) is an endemic salamander species to Iran, listed as “Critically Endangered” in the 2006 IUCN Red List due to population declines of 80%, over collection for the pet trade; area of occupancy less than 10 km2, fragmented populations, less than 1,000 adults, and continuing habitat degradation and loss. However, the Red List assessment was limited to surveys only around the type location of N. -

3Systematics and Diversity of Extant Amphibians
Systematics and Diversity of 3 Extant Amphibians he three extant lissamphibian lineages (hereafter amples of classic systematics papers. We present widely referred to by the more common term amphibians) used common names of groups in addition to scientifi c Tare descendants of a common ancestor that lived names, noting also that herpetologists colloquially refer during (or soon after) the Late Carboniferous. Since the to most clades by their scientifi c name (e.g., ranids, am- three lineages diverged, each has evolved unique fea- bystomatids, typhlonectids). tures that defi ne the group; however, salamanders, frogs, A total of 7,303 species of amphibians are recognized and caecelians also share many traits that are evidence and new species—primarily tropical frogs and salaman- of their common ancestry. Two of the most defi nitive of ders—continue to be described. Frogs are far more di- these traits are: verse than salamanders and caecelians combined; more than 6,400 (~88%) of extant amphibian species are frogs, 1. Nearly all amphibians have complex life histories. almost 25% of which have been described in the past Most species undergo metamorphosis from an 15 years. Salamanders comprise more than 660 species, aquatic larva to a terrestrial adult, and even spe- and there are 200 species of caecilians. Amphibian diver- cies that lay terrestrial eggs require moist nest sity is not evenly distributed within families. For example, sites to prevent desiccation. Thus, regardless of more than 65% of extant salamanders are in the family the habitat of the adult, all species of amphibians Plethodontidae, and more than 50% of all frogs are in just are fundamentally tied to water. -

Notes on the Distribution and Abundance of the Endangered Kaiser ’S Mountain Newt , Neurergus Kaiseri (C Audata : Salamandridae ), in Southwestern Iran
Herpetological Conservation and Biology 8(3):724−731. HSuebrpmeittotelodg: i1c aJlu Clyo 2n0se1r2v;a Aticocne apntedd B: 9io Jlouglyy 2013; Published: 31 December 2013. Notes oN the DistributioN aND abuNDaNce of the eNDaNgereD Kaiser ’s MouNtaiN Newt , Neurergus kaiseri (c auData : salaMaNDriDae ), iN southwesterN iraN Mozafar sharifi 1* , h osseiN farasat 1, h osseiN BaraNi -B eiraNv 2, soMaye vaissi 1, and ehsaN foroozaNfar 3 1Razi University Center for Environmental Studies, Department of Biology, Faculty of Sciences, Kermanshah 67149, Iran 2Department of Biology, Faculty of Sciences, Ferdowsi University of Mashhad, Mashhad, Iran 3Faculty of Environment, Ostandari Square, Karaj, Iran *Corresponding author, e-mail: [email protected] abstract. —the endangered Kaiser’s Mountain Newt, Neurergus kaiseri , is a species endemic to the southern Zagros range in iran. until now, N. kaiseri had been reported from only five localities. the present study describes eight new localities that increase the species’ known range from 212 km 2 (minimum convex polygon) to 789 km 2 at elevations between 930−1395 meters. the localities are generally dispersed; nearest neighbor distances among the 13 localities average 4.61 km (range, 0.93−12.39 km). the streams are separated from one another by steep and rocky terrain with vegetation cover of mature open oak woodland in the west and sparse scrubland or thin oak-pistachio woodland in the central area and east, thus potentially isolating many of the populations from each other. we surveyed 12 of the 13 localities with newts 1−2 times and counted a total of 1,277 adults, post-metamorphic sub-adults, and larvae (mean per stream, 106; range, 2−650) in a total of 4.23 km of stream reaches. -

Feeding in Amphibians: Evolutionary Transformations and Phenotypic Diversity As Drivers of Feeding System Diversity
Chapter 12 Feeding in Amphibians: Evolutionary Transformations and Phenotypic Diversity as Drivers of Feeding System Diversity Anthony Herrel, James C. O’Reilly, Anne-Claire Fabre, Carla Bardua, Aurélien Lowie, Renaud Boistel and Stanislav N. Gorb Abstract Amphibians are different from most other tetrapods because they have a biphasic life cycle, with larval forms showing a dramatically different cranial anatomy and feeding strategy compared to adults. Amphibians with their exceptional diversity in habitats, lifestyles and reproductive modes are also excellent models for studying the evolutionary divergence in feeding systems. In the present chapter, we review the literature on amphibian feeding anatomy and function published since 2000. We also present some novel unpublished data on caecilian feeding biome- chanics. This review shows that over the past two decades important new insights in our understanding of amphibian feeding anatomy and function have been made possible, thanks to a better understanding of the phylogenetic relationships between taxa, analyses of development and the use of biomechanical modelling. In terms of functional analyses, important advances involve the temperature-dependent nature of tongue projection mechanisms and the plasticity exhibited by animals when switch- A. Herrel (B) Département Adaptations du Vivant, Muséum national d’Histoire naturelle, UMR 7179 C.N.R.S/M.N.H.N, 55 rue Buffon, 75005, Paris Cedex 05, France e-mail: [email protected] J. C. O’Reilly Department of Biomedical Sciences, Ohio University, Cleveland Campus, Cleveland, Ohio 334C, USA A.-C. Fabre · C. Bardua Life Sciences Department, The Natural History Museum, Cromwell Road, London SW7 5BD, UK A. Lowie Department of Biology Evolutionary, Morphology of Vertebrates, Ghent University, K.L.