Camera Traps Unravel the Effects of Weather Conditions and Predator Presence on the Activity Levels of Two Lizards
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

Sexual Selection and Signalling in the Lizard Platysaurus Minor
SEXUAL SELECTION AND SIGNALLING IN THE LIZARD PLATYSAURUS MINOR Belinda Ann Lewis A dissertation submitted to the Faculty of Science, University of the Witwatersrand, Johannesburg, in fulfilment of the requirements for the degree of Master of Science. Johannesburg, 2006 I declare that this dissertation is my own unaided work. It is being submitted for the degree of Master of Science in the University of the Witwatersrand, Johannesburg. It has not been submitted before for any degree or examination in any other University. ________________________ Belinda A. Lewis 6th day of December 2006 i ABSTRACT Sexual selection may influence aspects of male morphology associated with territoriality, female choice, aggression and contest success. Attributes that are most commonly selected for include body size, condition, weaponry, endurance and bright coloration. I investigated the relationships between morphology, use of space and home range quality, and access to females. Specifically, I examined the relationships between colour, body size and condition, and whether morphology could predict aggression or contest success. Colour spectral data were analyzed using both traditional measures of colour (hue, chroma, brightness) and principal components. Males with darker, more saturated chests, and more saturated throats, had larger home ranges. Home range quality, as determined by refuge number and prey availability, was associated with blue chests and blue throats and chests, respectively. Males with larger home ranges had higher numbers of associated females and spent more time courting females. Larger males in better condition had darker, more saturated chests. Males in better body condition were also more aggressive. There was a consistent trend for larger males to win more contests, but this relationship was only significant in analyses using traditional measures of colour. -

Morphological and Ecological Correlates of Bite Force in the Rockdwelling Lizards Ouroborus Cataphractus And
bs_bs_banner Biological Journal of the Linnean Society, 2014, 111, 823–833. With 4 figures Under pressure: morphological and ecological correlates of bite force in the rock-dwelling lizards Ouroborus cataphractus and Karusasaurus polyzonus (Squamata: Cordylidae) Downloaded from https://academic.oup.com/biolinnean/article/111/4/823/2415797 by guest on 28 September 2021 CHRIS BROECKHOVEN* and P. LE FRAS N. MOUTON Department of Botany & Zoology, Stellenbosch University, Private Bag X1, Matieland 7602, Stellenbosch, South Africa Received 31 October 2013; revised 2 December 2013; accepted for publication 2 December 2013 Rock-dwelling lizards are hypothesized to be highly constrained in the evolution of head morphology and, consequently, bite force. Because the ability to generate a high bite force might be advantageous for a species’ dietary ecology, morphological changes in head configuration that allow individuals to maintain or improve their bite force under the constraint of crevice-dwelling behaviour are to be expected. The present study addressed this issue by examining head morphology, bite force, and a number of dietary traits in the rock-dwelling cordylid lizards Ouroborus cataphractus and Karusasaurus polyzonus. The results obtained show that O. cataphractus has a larger head and higher bite force than K. polyzonus. In K. polyzonus, head width, lower jaw length, and jaw closing-in lever are the best predictors of bite force, whereas head height is the main determinant of bite force in O. cataphractus. Although the observed difference in bite force between the species does not appear to be related to dietary patterns or prey handling, the prey spectrum available for intake was greater in O. -

A Phylogeny and Revised Classification of Squamata, Including 4161 Species of Lizards and Snakes
BMC Evolutionary Biology This Provisional PDF corresponds to the article as it appeared upon acceptance. Fully formatted PDF and full text (HTML) versions will be made available soon. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes BMC Evolutionary Biology 2013, 13:93 doi:10.1186/1471-2148-13-93 Robert Alexander Pyron ([email protected]) Frank T Burbrink ([email protected]) John J Wiens ([email protected]) ISSN 1471-2148 Article type Research article Submission date 30 January 2013 Acceptance date 19 March 2013 Publication date 29 April 2013 Article URL http://www.biomedcentral.com/1471-2148/13/93 Like all articles in BMC journals, this peer-reviewed article can be downloaded, printed and distributed freely for any purposes (see copyright notice below). Articles in BMC journals are listed in PubMed and archived at PubMed Central. For information about publishing your research in BMC journals or any BioMed Central journal, go to http://www.biomedcentral.com/info/authors/ © 2013 Pyron et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes Robert Alexander Pyron 1* * Corresponding author Email: [email protected] Frank T Burbrink 2,3 Email: [email protected] John J Wiens 4 Email: [email protected] 1 Department of Biological Sciences, The George Washington University, 2023 G St. -

Observations of Infanticide and Cannibalism in Four Species of Cordylid Lizard (Squamata: Cordylidae) in Captivity and the Wild
Herpetology Notes, volume 14: 725-729 (2021) (published online on 21 April 2021) Observations of infanticide and cannibalism in four species of cordylid lizard (Squamata: Cordylidae) in captivity and the wild Daniel van Blerk1,†, Jens Reissig2,†, Julia L. Riley 3,†, John Measey1,*, and James Baxter-Gilbert1 Cannibalism, the consumption of conspecifics, of Africa (Reissig, 2014), from Ethiopia to South Africa is taxonomically widespread and occurs across a (latitudinally) and Angola to Ethiopia (longitudinally). diversity of reptilian species (Polis and Myers, 1985). Here, we present observations of cannibalism by four A long-standing, yet antiquated, perspective views species of cordylid lizard, two from free-living wild cannibalism as an aberrant behaviour (as discussed populations and another two from captive settings. Since in Fox, 1975), but contemporary investigations have the natural history of many cordylid species remains noted its important role in the ecology and evolution of deficient, and several species have been observed to many wild populations (Robbins et al., 2013; Cooper display reasonably high degrees of sociality, like group- et al., 2015; Van Kleek et al., 2018). Examples of this living in Armadillo Lizards, Ouroborus cataphractus include habitat partitioning and optimising resource (Boie, 1828) (Mouton, 2011) and Sungazers, Smaug availability, as seen in juvenile Komodo Dragons, giganteus (Smith, 1844) (Parusnath, 2020), these Varanus komodoensis Ouwens, 1912, taking to the trees observations provide important insights -
Johan Marais
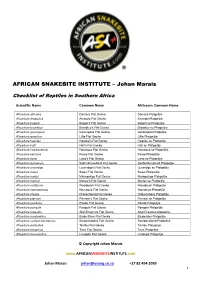
AFRICAN SNAKEBITE INSTITUTE – Johan Marais Checklist of Reptiles in Southern Africa Scientific Name Common Name Afrikaans Common Name Afroedura africana Damara Flat Gecko Damara Platgeitjie Afroedura amatolica Amatola Flat Gecko Amatola Platgeitjie Afroedura bogerti Bogert's Flat Gecko Bogert se Platgeitjie Afroedura broadleyi Broadley’s Flat Gecko Broadley se Platgeitjie Afroedura gorongosa Gorongosa Flat Gecko Gorongosa Platgeitjie Afroedura granitica Lillie Flat Gecko Lillie Platgeitjie Afroedura haackei Haacke's Flat Gecko Haacke se Platgeitjie Afroedura halli Hall's Flat Gecko Hall se Platgeitjie Afroedura hawequensis Hawequa Flat Gecko Hawequa se Platgeitjie Afroedura karroica Karoo Flat Gecko Karoo Platgeitjie Afroedura langi Lang's Flat Gecko Lang se Platgeitjie Afroedura leoloensis Sekhukhuneland Flat Gecko Sekhukhuneland Platgeitjie Afroedura loveridgei Loveridge's Flat Gecko Loveridge se Platgeitjie Afroedura major Swazi Flat Gecko Swazi Platgeitjie Afroedura maripi Mariepskop Flat Gecko Mariepskop Platgeitjie Afroedura marleyi Marley's Flat Gecko Marley se Platgeitjie Afroedura multiporis Woodbush Flat Gecko Woodbush Platgeijtie Afroedura namaquensis Namaqua Flat Gecko Namakwa Platgeitjie Afroedura nivaria Drakensberg Flat Gecko Drakensberg Platgeitjie Afroedura pienaari Pienaar’s Flat Gecko Pienaar se Platgeitjie Afroedura pondolia Pondo Flat Gecko Pondo Platgeitjie Afroedura pongola Pongola Flat Gecko Pongola Platgeitjie Afroedura rupestris Abel Erasmus Flat Gecko Abel Erasmus platgeitjie Afroedura rondavelica Blyde River -

Patterns of Species Richness, Endemism and Environmental Gradients of African Reptiles
Journal of Biogeography (J. Biogeogr.) (2016) ORIGINAL Patterns of species richness, endemism ARTICLE and environmental gradients of African reptiles Amir Lewin1*, Anat Feldman1, Aaron M. Bauer2, Jonathan Belmaker1, Donald G. Broadley3†, Laurent Chirio4, Yuval Itescu1, Matthew LeBreton5, Erez Maza1, Danny Meirte6, Zoltan T. Nagy7, Maria Novosolov1, Uri Roll8, 1 9 1 1 Oliver Tallowin , Jean-Francßois Trape , Enav Vidan and Shai Meiri 1Department of Zoology, Tel Aviv University, ABSTRACT 6997801 Tel Aviv, Israel, 2Department of Aim To map and assess the richness patterns of reptiles (and included groups: Biology, Villanova University, Villanova PA 3 amphisbaenians, crocodiles, lizards, snakes and turtles) in Africa, quantify the 19085, USA, Natural History Museum of Zimbabwe, PO Box 240, Bulawayo, overlap in species richness of reptiles (and included groups) with the other ter- Zimbabwe, 4Museum National d’Histoire restrial vertebrate classes, investigate the environmental correlates underlying Naturelle, Department Systematique et these patterns, and evaluate the role of range size on richness patterns. Evolution (Reptiles), ISYEB (Institut Location Africa. Systematique, Evolution, Biodiversite, UMR 7205 CNRS/EPHE/MNHN), Paris, France, Methods We assembled a data set of distributions of all African reptile spe- 5Mosaic, (Environment, Health, Data, cies. We tested the spatial congruence of reptile richness with that of amphib- Technology), BP 35322 Yaounde, Cameroon, ians, birds and mammals. We further tested the relative importance of 6Department of African Biology, Royal temperature, precipitation, elevation range and net primary productivity for Museum for Central Africa, 3080 Tervuren, species richness over two spatial scales (ecoregions and 1° grids). We arranged Belgium, 7Royal Belgian Institute of Natural reptile and vertebrate groups into range-size quartiles in order to evaluate the Sciences, OD Taxonomy and Phylogeny, role of range size in producing richness patterns. -

Causes and Consequences of Body Armour in the Group-Living Lizard, Ouroborus Cataphractus (Cordylidae)
Causes and consequences of body armour in the group-living lizard, Ouroborus cataphractus (Cordylidae) by Chris Broeckhoven Dissertation presented for the degree of Doctor of Philosophy in the Faculty of Science at Stellenbosch University Supervisor: Prof. P. le Fras N. Mouton March 2015 Stellenbosch University https://scholar.sun.ac.za DECLARATION By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. March 2015 Copyright © 2015 Stellenbosch University All rights reserved i Stellenbosch University https://scholar.sun.ac.za ABSTRACT Cordylidae is a family of predominantly rock-dwelling sit-and-wait foraging lizards endemic to southern Africa. The significant variation in spine length and extent of osteoderms among taxa makes the family an excellent model system for studying the evolution of body armour. Specifically, the Armadillo lizard (Ouroborus cataphractus) offers an ideal opportunity to investigate the causes and consequences of body armour. Previous studies have hypothesised that high terrestrial predation pressure, resulting from excursions to termite foraging ports away from the safety of the shelter, has led to the elaboration of body armour and a unique tail-biting behaviour. The reduction in running speed associated with heavy body armour, in turn, appears to have led to the evolution of group-living behaviour to lower the increased aerial predation risk. -

A Taxonomic Revision of the South-Eastern Dragon Lizards of the Smaug Warreni (Boulenger) Species Complex in Southern Africa, Wi
A taxonomic revision of the south-eastern dragon lizards of the Smaug warreni (Boulenger) species complex in southern Africa, with the description of a new species (Squamata: Cordylidae) Michael F. Bates1,2,* and Edward L. Stanley3,* 1 Department of Herpetology, National Museum, Bloemfontein, South Africa 2 Department of Zoology and Entomology, University of the Free State, Bloemfontein, South Africa 3 Department of Herpetology, Florida Museum of Natural History, Gainesville, FL, USA * These authors contributed equally to this work. ABSTRACT A recent multilocus molecular phylogenyofthelargedragonlizardsofthegenus Smaug Stanley et al. (2011) recovered a south-eastern clade of two relatively lightly- armoured, geographically-proximate species (Smaug warreni (Boulenger, 1908) and S. barbertonensis (Van Dam, 1921)). Unexpectedly, S. barbertonensis was found to be paraphyletic, with individuals sampled from northern Eswatini (formerly Swaziland) being more closely related to S. warreni than to S. barbertonensis from the type locality of Barberton in Mpumalanga Province, South Africa. Examination of voucher specimens used for the molecular analysis, as well as most other available museum material of the three lineages, indicated that the ‘Eswatini’ lineage—including populations in a small area on the northern Eswatini–Mpumalanga border, and northern KwaZulu–Natal Province in South Africa—was readily distinguishable from S. barbertonensis sensu stricto (and S. warreni) by its unique dorsal, lateral and ventral colour patterns. In order to further assess the taxonomic status of the three populations, a detailed Submitted 26 September 2019 Accepted 7 January 2020 morphological analysis was conducted. Multivariate analyses of scale counts and Published 25 March 2020 body dimensions indicated that the ‘Eswatini’ lineage and S. -

ROMANSRIVIER (ESKOM) POWERLINE ROUTE: Baseline Assessment of Mammals, Amphibians and Reptiles
ROMANSRIVIER (ESKOM) POWERLINE ROUTE: Baseline assessment of mammals, amphibians and reptiles Report compiled for: SRK Consulting Client: Eskom Report compiled by: Marius Burger, trading as Sungazer, 6 Putter Street, Lakeside 7945 Phone: 083-2317452; Email: [email protected] FINAL DRAFT – September 2017 Figure 1: The proposed route for the Romansrivier powerline, with the black-centred yellow circles (1-67) indicating the pylon positions. DECLARATION OF INDEPENDENCE I hereby declare that I have no conflicts of interest related to the work of this report. Specifically, I declare that I have no personal financial interests in the property and/or development being assessed in this report, and that I have no personal or financial connections to the relevant property owners, developers, planners, financiers or consultants of the development. I declare that the opinions expressed in this report are my own and a true reflection of my professional expertise. CV OF SPECIALIST CONSULTANT (abridged) Mr Marius Burger holds a National Diploma in Nature Conservation with Cape Technicon, and worked as a research assistant with Eastern Cape Nature Conservation (1987-1997). Subsequently he took up employment with the Animal Demography Unit (ADU, University of Cape Town) as National Coordinator of the Southern African Frog Atlas Project (1997-2003) and as Project Herpetologist of the Southern African Reptile Conservation Assessment (2005-2009). Burger’s EIA activities as a faunal specialist started in 1996, and since then he has participated in about 85 different projects in collaboration with a variety of EIA consultancies. In 1998, he established a sole-proprietor business Sungazer. His achievements as a faunal specialist are summarised below: Research collaborator with FLORA FAUNA & MAN, Ecological Services Ltd.: 2011 – present. -

Population Genetics and Sociality in the Sungazer (Smaug Giganteus)
Population genetics and sociality in the Sungazer (Smaug giganteus) Shivan Parusnath Doctoral Thesis Supervisors: Prof. Graham Alexander Prof. Krystal Tolley Prof. Desire Dalton Prof. Antoinette Kotze A thesis submitted to the Faculty of Science, University of the Witwatersrand, Johannesburg, in fulfilment of the requirements for the degree of Doctor of Philosophy. 30th October 2020 “My philosophy is basically this, and this is something that I live by, and I always have, and I always will: Don't ever, for any reason, do anything, to anyone, for any reason, ever, no matter what, no matter where, or who, or who you are with, or where you are going, or where you've been, ever, for any reason whatsoever.” - Michael G. Scott DECLARATION I declare that this thesis is my own, unaided work. It is being submitted for the Degree of Doctor of Philosophy at the University of the Witwatersrand, Johannesburg. It has not been submitted before for any degree or examination at any other University. All protocols were approved by the University of the Witwatersrand Animal Ethics Screening Committee (2014/56/B; 2016/06/30/B), and the National Zoological Garden, South African National Biodiversity Institute (NZG SANBI) Research, Ethics and Scientific Committee (P14/18). Permits to work with Threatened or Protected Species were granted by the South African Department of Environmental Affairs (TOPS59). Permits to collect samples in the Free State and Mpumalanga provinces were provided by Department of Small Business Development, Tourism and Environmental Affairs (01/34741; JM938/2017) and Mpumalanga Tourism and Parks Agency (MPB. 5519) respectively. Research approval in terms of Section 20 of the Animal Diseases Act (35 of 1984) was granted by the South African Department of Agriculture, Forestry and Fisheries (12/11/1/1/18). -

Potential Invasion Risk of Pet Traded Lizards, Snakes, Crocodiles
diversity Article Potential Invasion Risk of Pet Traded Lizards, Snakes, Crocodiles, and Tuatara in the EU on the Basis of a Risk Assessment Model (RAM) and Aquatic Species Invasiveness Screening Kit (AS-ISK) OldˇrichKopecký *, Anna Bílková, Veronika Hamatová, Dominika K ˇnazovická, Lucie Konrádová, Barbora Kunzová, Jana Slamˇeníková, OndˇrejSlanina, Tereza Šmídová and Tereza Zemancová Department of Zoology and Fisheries, Faculty of Agrobiology, Food and Natural Resources, Czech University of Life Sciences Prague, Kamýcká 129, Praha 6 - Suchdol 165 21, Prague, Czech Republic; [email protected] (A.B.); [email protected] (V.H.); [email protected] (D.K.); [email protected] (L.K.); [email protected] (J.S.); [email protected] (B.K.); [email protected] (O.S.); [email protected] (T.S.); [email protected] (T.Z.) * Correspondence: [email protected]; Tel.: +420-22438-2955 Received: 30 June 2019; Accepted: 9 September 2019; Published: 13 September 2019 Abstract: Because biological invasions can cause many negative impacts, accurate predictions are necessary for implementing effective restrictions aimed at specific high-risk taxa. The pet trade in recent years became the most important pathway for the introduction of non-indigenous species of reptiles worldwide. Therefore, we decided to determine the most common species of lizards, snakes, and crocodiles traded as pets on the basis of market surveys in the Czech Republic, which is an export hub for ornamental animals in the European Union (EU). Subsequently, the establishment and invasion potential for the entire EU was determined for 308 species using proven risk assessment models (RAM, AS-ISK). Species with high establishment potential (determined by RAM) and at the same time with high potential to significantly harm native ecosystems (determined by AS-ISK) included the snakes Thamnophis sirtalis (Colubridae), Morelia spilota (Pythonidae) and also the lizards Tiliqua scincoides (Scincidae) and Intellagama lesueurii (Agamidae).