Final Report Dec 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WINE Talk: October 2016
Licence No 58292 30 Salamanca Square, Hobart GPO Box 2160, Hobart Tasmania, 7001 Australia Telephone +61 3 6224 1236 [email protected] www.livingwines.com.au WINE Talk: October 2016 The newsletter of Living Wines: Edition 65 Since the last newsletter we have travelled to Melbourne and Geelong to conduct wine tastings and also to Sydney to attend the high-energy Mental Notes event which, this year, was held at the Paddington Town Hall – an excellent venue for an event of this type. We also joined with a number of our other colleagues from this event to hold a trade tasting at The Bentley Bar and Restaurant on the Monday following Mental Notes. We are now gearing up for the other two major events on the natural wine calendar – Rootstock in Sydney and Soul For Wine in Melbourne. We are delighted that both Tony and Philippe Bornard will be attending Rootstock this year. To celebrate their visit we will be holding two very special events. The first will be a late lunch in Hobart at Franklin Restaurant and the second will be a dinner at the wonderful Bar Brosé in Sydney which will be cooked by Analeise Gregory as described below. And now to the special packs. We have a rather long story on the Côte de Beaune and a 6 pack of wines selected from that region of Burgundy. We have a special dozen comprised of wines that include very rare grape varieties that we like to seek out from the hidden corners of France. There is a pack of wines from our winemaker of the month, namely Domaine les Grandes Vignes from the Loire Valley. -

Vitis International Variety Catalogue Passport Data
Vitis International Variety Catalogue www.vivc.de Passport data Prime name PINOT BLANC Color of berry skin BLANC Variety number VIVC 9272 Country or region of origin of the variety FRANCE Species VITIS VINIFERA LINNÉ SUBSP. VINIFERA Pedigree as given by breeder/bibliography PINOT NOIR MUTATION Pedigree confirmed by markers Full pedigree NO Prime name of parent 1 Prime name of parent 2 Parent - offspring relationship Offspring YES Breeder Breeder institute code Breeder contact address Year of crossing Year of selection Year of protection Formation of seeds COMPLETE Sex of flowers HERMAPHRODITE Taste NONE Chlorotype A Photos of the cultivar 13 SSR-marker data YES Loci for resistance Degree of resistance YES Loci of traits Table of accession names YES Table of area YES Registered in the European Catalogue YES Links to: - Bibliography - Remarks to prime names and institute codes September 25, 2021 © Institute for Grapevine Breeding - Geilweilerhof 1 Julius Kühn-Institut Vitis International Variety Catalogue www.vivc.de Synonyms: 99 AG PINO ARBST WEISS ARNAISON BLANC ARNOISON AUVERNAS AUVERNAT AUVERNAT BLANC AUXERROIS BELI PINOT BEYAZ BURGUNDER BIELA KLEVANJKA BIJELI PINO BLANC DE CHAMPAGNE BON BLANC BORGOGNA BIANCA BORGOGNA BIANCO BORGOGNINO BORGONA BLANCO BORGONJA BELA MALA BORONJA MALO ZRNO BURGUNDA BURGUNDAC BURGUNDAC BELI BURGUNDAC BIJELI BURGUNDER BLANC BURGUNDER WEISS BURGUNDER WEISSER BURGUNDI FEHER BURGUNDI KISFEHER BURGUNDISCHE BURGUNDSKE BIELI BURGUNDSKE BILE XIMENESTRAUBE CHARDONNET PINOT BLANC CLAEVNER CLEVNER DAUNE EPINETTE EPINETTE -

Wine Talk: August 2013
Licence No 58292 30 Salamanca Square, Hobart GPO Box 2160, Hobart Tasmania, 7001 Australia Telephone +61 3 6224 1236 [email protected] www.livingwines.com.au Wine Talk: August 2013 The newsletter of Living Wines: Edition 38 This month we have seven special packs for you, including two packs of hard-to- find magnums that we have in stock. There are only a few of the magnum packs so we expect them to disappear quite quickly. We have been watching the stocks of Domaine de l’Octavin and Mylène Bru, which arrived recently disappearing out the door. We are also pleased to announce the imminent arrival of another new producer – this time from the Roussillon area close to the Spanish border. We have been lucky to be able to offer the fascinating wines from Jolly Ferriol who produce interesting white and red wines as well as traditional lines such as Muscat de Rivesaltes. They also do a great Pet Nat of which we will receive good stocks and a very rare rancio of which we’ll have hardly any stocks! But back to the Special Packs. The first is from our most recent arrivals from Domaine de l’Octavin in the Jura and Mylène Bru in the Languedoc. These wines (especially the Octavin) are in very short supply so this is probably the only opportunity for our newsletter subscribers to try some of their offerings. We have also put together a six pack that features the Grenache grape with some very interesting wines showing the many facets of this grape variety. -

Vitis International Variety Catalogue \(VIVC\)
BIO Web of Conferences 5, 01009 (2015) DOI: 10.1051/bioconf/20150501009 © Owned by the authors, published by EDP Sciences, 2015 Vitis International Variety Catalogue (VIVC): A cultivar database referenced by genetic profiles and morphology Erika Maul and Reinhard Töpfer Julius Kühn Institut, Institut für Rebenzüchtung Geilweilerhof, 76833 Siebeldingen, Germany Abstract. The establishment of the Vitis International Variety Catalogue (VIVC) dates back to 1984. The idea was to virtually assemble all accessions maintained in the worldwide existing collections to face genetic erosion. In many cases synonymy, homonymy and misnaming hampered the clear assignment of cultivars/accessions to prime names. In the past 15 years nuclear microsatellites, in particular the nine SSR-markers VVS2, VVMD5, VVMD7, VVMD25, VVMD27, VVMD28, VVMD32, VrZAG62 and VrZAG79 were extensively applied for cultivar recognition in combination with ampelography. Genetic fin- gerprints of more than 15,000 cultivars/accessions were collected. They were taken from more than 300 articles and from microsatellite databases on the web. Allele sizes were adapted according to own internal reference varieties. Comparison of profiles revealed new identities like: “Corbeau” = “Sevilhao”, “Gragnelut” = “Fer”, “Beretinjak” = “Bianco d’Alessano”. The activities aim to equip the prime names of VIVC with reliable genetic profiles combined with the validation of their identity by ampelography. Fingerprints from 1,500 cultivars were already uploaded in VIVC. Two distinct search modules were imple- mented: “Microsatellites by varieties” and “Microsatellites by profiles”. The implementation assists the management of grape- vine genetic resources, e.g. trueness to type assessment in grapevine collections and serves research and breeding. Due to the large anthropogenic spread of grapevines it is 1. -

Wine Talk 58 January 2016
Licence No 58292 30 Salamanca Square, Hobart GPO Box 2160, Hobart Tasmania, 7001 Australia Telephone +61 3 6224 1236 [email protected] www.livingwines.com.au WINE Talk: January 2016 The newsletter of Living Wines: Edition 58 Welcome to the first Wine Talk newsletter for 2016. We are looking forward to a busy year ahead with the beautiful wines made from the exciting 2015 harvest starting to arrive over the coming months. This month we have a short feature about one of our long-term suppliers Stéphane Guion from the Loire Valley. His wines often slip under the radar due to their low price, but they are very well-made wines made from Cabernet Franc. There is no special offer because the wines are offered at such a low price, but it is a good chance to buy a dozen which we will ship freight free. We have seven special packs for you this month. We have decided to create a mixed 12 pack again this month with a 20% discount and free freight due to the amazing reaction to last month’s special offer. The rest of the packs have a 15% discount. We have a special Gamay 6 pack with wines from Beaujolais and from the Loire Valley. We are also featuring the wonderful sparkling wines created by “petillant naturel” specialist Les Capriades. We follow these up with a 3 pack of Magnums and a 3 pack of lovely Champagnes, all from the same producer but from different plots and made with different grapes. We also have assembled a pack of reds that are perfect for drinking chilled. -
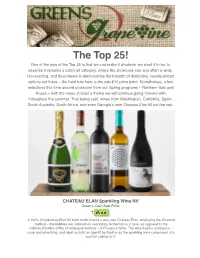
The Top 25! One of the Joys of the Top 25 Is That We Can Make It Whatever We Want It to Be
The Top 25! One of the joys of the Top 25 is that we can make it whatever we want it to be. In essence it remains a catch-all category, where the showcase can and often is wide, far-reaching, and thus meant to demonstrate the breadth of distinctive, keenly-priced options out there – the hard rule here is the sub-$10 price point. Nonetheless, a few selections this time around crossover from our Spring programs – Northern Italy and Roses – with the roses at least a theme we will continue going forward with throughout the summer. That being said, wines from Washington, California, Spain, South Australia, South Africa, and even Georgia’s own Chateau Elan fill out the rest. CHATEAU ELAN Sparkling Wine NV Green’s Cash Sale Price: A 100% Chardonnay Brut NV from north Atlanta’s very own Chateau Elan, employing the Charmat method – the bubbles are induced via secondary fermentation in tank, as opposed to the individual bottles of the Champagne method – of Prosecco fame. The wine itself is analogous – crisp and refreshing, and ideal as both an aperitif by itself or as the sparkling wine component of a cocktail calling for it. DANZANTE Pinot Grigio 2014 Green’s Cash Sale Price: A textbook northern Italian-style Pinot Grigio from the Tre Venezie IGT. Light-bodied, with delicate fruit notes of pear, green apple and citrus peel complement the crisp acidity and mineral finish – for many a Pinot Grigio drinker, this is THE style of Pinot Grigio. DOUGLAS GREEN Cabernet Sauvignon 2013 Green’s Cash Sale Price: A 100% Cabernet Sauvignon from one of South Africa’s first “negociants”, the fruit here is sourced from the Swartland & Robertson regions of the country’s southwest corner. -

California Grape Varieties
California Grape Varieties The following is a list of California grape varieties that are available each fall starting in September. RED GRAPE VARIETIES ALICANTE BOUSHET Alicante Bouschet is a wine grape variety that has been widely cultivated since 1866. It is a cross of Grenache with Petit Bouschet (itself a cross of the very old variety Teinturier du Cher and Aramon). Alicante is a teinturier, a grape with red flesh. It is the only teinturier grape that belongs to the Vitis vinifera family. It’s deep color makes it useful for blending with light red wine. It was planted heavily during Prohibition in California for export to the East Coast. This grape makes a dry, dark, full bodied wine. BARBERA Barbera is believed to have originated in the hills of Monferrato in central Piemonte, Italy where it has been known from the thirteenth century. In California, Barbera is one of the most successful of the Piemontese grapes to be adapted in the state, with over 8000 acres of plantings. This grape produces a red, deep colored, full bodied wine and produces a dry somewhat tannic wine that ages well and softens with time. When young, the wines offer a very intense aroma of fresh red and blackberries. In the lightest versions notes of cherries, raspberries and blueberries and with notes of blackberry and black cherries in wines made of more ripe grapes. Oaking this wine provides for increased complexity, aging potential, and hints of vanilla notes. BLACK MALVASIA The Malvasia family of grapes are of ancient origin, most likely originating in Greece, but now grown in many of the winemaking regions of the world. -

Seven-Page Article
JANUARY 2019 JANUARY WINEJanuary 2019 • $5.95 BUSINESSThe Industry’s Leading Publication for Wineries and GrowersMONTHLY www.winebusiness.com Now Incorporating WINE BUSINESS MONTHLY Vineyardocus: Comparing Different WinemakingF Regimens for a Single Vineyard V F V y • 2019 UNIFIED GUIDE 2019 UNIFIED GUIDE A preview of the Unified Wine & Grape Symposium For more on Sauvignon Blanc, see the winemaking VARIETAL FOCUS in the January 2017 issue of WINE BUSINESS MONTHLY www.winebusiness.com/wbm Influences on Sauvignon Blanc Style Explored at Lake County International Symposium Ted Rieger Ted Rieger, CSW, is a wine journalist based in Sacramento, California and a writer for wine trade media since 1988. BILL GROODY THE 2018 INTERNATIONAL SAUVIGNON Blanc Symposium held The event prominently featured Lake County producers and their wines, last spring in Lake County, California provided a comprehensive overview but also included producers from New Zealand and coastal California from experts in climatology, soils and plant/clonal material, and interna- regions. An overarching theme was that, although Sauvignon Blanc is one tional and local viticulturists and winemakers all focused on the production of the most distinctive and identifiable wines in aroma and flavor, it can of Sauvignon Blanc. Hosted and organized by the Lake County Winegrape be made in a wide range of styles by enhancing and managing aroma and Commission (LCWC) and held at Chacewater Winery and Olive Mill in flavor chemistry in the vineyard and in the winery. Presentations included Kelseyville, this is the third such symposium hosted by the LCWC since 2002. the results of research trials accompanied by tastings to compare Sauvignon Wine journalist and wine educator Deborah Parker Wong was moderator Blanc produced from different clones, from different appellations, picked at for the day-long meeting. -

WINE Talk: June 2017
Licence No 58292 30 Salamanca Square, Hobart GPO Box 2160, Hobart Tasmania, 7001 Australia Telephone +61 3 6224 1236 [email protected] www.livingwines.com.au WINE Talk: June 2017 The newsletter of Living Wines: Edition 69 Welcome to the 69th edition of Wine Talk. We have the second story about how humans perceive taste and aroma with a lot of information about the latest scientific research into this fascinating area. If you missed the story in the last newsletter it is available on the Living Wines website in the Information section. We also have included some information about some imminent arrivals including the much sought-after wines of Renaud Bruyère and Adeline Houillon from the Jura. We have also written about the success of the Bottletops event in Hobart which saw a stellar line-up of local and interstate natural winemakers displaying their latest wines for the public. We also have an interesting collection of packs for you this month, headlined by a pack of new arrivals from a special new producer sin the Languedoc, Opi d’Aqui (not to be confused with the beautiful wines from Es d’Aqui imported by our friends at Lo-Fi Wines). You can read more about the light, almost ethereal wines of Opi d’Aqui in the description of Pack 1 below. We have also created a special pack of white wines including some that are in very short supply. We have often written about our affection for Carignan so we have put together a pack of wines created from this southern grape variety. -

From Pinot to Xinomavro in the World's Future Wine-Growing Regions
PERSPECTIVE https://doi.org/10.1038/s41558-017-0016-6 From Pinot to Xinomavro in the world's future wine-growing regions E. M. Wolkovich 1,2*, I. García de Cortázar-Atauri3, I. Morales-Castilla1,2, K. A. Nicholas 4 and T. Lacombe 5 Predicted impacts of climate change on crops—including yield declines and loss of conservation lands—could be mitigated by exploiting existing diversity within crops. Here we examine this possibility for wine grapes. Across 1,100 planted varieties, wine grapes possess tremendous diversity in traits that affect responses to climate, such as phenology and drought tolerance. Yet little of this diversity is exploited. Instead many countries plant 70–90% of total hectares with the same 12 varieties—repre- senting 1% of total diversity. We outline these challenges, and highlight how altered planting practices and new initiatives could help the industry better adapt to continued climate change. limate change poses a major challenge to agriculture. New One major perennial crop that has high existing diversity and temperature and precipitation regimes, including new appears well adapted to handle shifting climate is common grape- extremes in heat and drought1, are dramatically changing the vine (Vitis vinifera subsp. vinifera). Currently at least 6,000 culti- C 2 climate of most agricultural areas . Even if warming is kept to the vated varieties of V. vinifera are planted across the globe for research Paris Agreement target of “well below 2.0°C,” almost all agricultural or production19 and this genotypic variation is linked to high varia- land will continue to experience large shifts in the local climate for tion in a number of characteristics, including cold tolerance, ripen- decades to come. -

The Riesling Predicament
ONE The Riesling Predicament very very good wine is a trifecta of gated by human intervention. Varieties have E variety, place, and style. Variety, of course, been with us more or less as long as people refers to the wine’s varietal composition. Place have made wine and cultivated grapes, but they denotes the wine’s geographic origin. And style were not an object of systematic attention or a is shorthand for most other factors, such as key element in wine nomenclature until fairly whether the wine was made still or sparkling, recently. Familiar as varietal names now appear heady or light, sweet or dry. These three param- to wine consumers around the world, ranging eters are kaleidoscopically interwoven and sub- through the alphabet from Albariño to Zinfan- tly interdependent, but in the end they all deter- del, most varieties were not segregated in vine- mine a wine’s individual properties—from yards, made monovarietally, or featured on color, strength, structure, taste, and smell to wine labels until the past 150 or so years. And reputation, cost, price, and suitability. the science that has finally begun to make The pages that follow discuss one wine sense of the large universe of varieties, and to grape variety, Riesling, made as monovarietal reveal parental and sibling relationships among wine, grown in dozens of different sites across them, developed only in the 1990s. Today at the Northern Hemisphere, and vinified so that least 1,400 wine grape varieties are known to the finished wine is dry. Before we begin this be present in the world’s commercial vineyards, exploration of specifics, a bit more attention to and each has been genetically fingerprinted the wine’s background is appropriate. -

FINAL Alta Luna Bottle Basics
PASSION INTEGRITY FAMILY CURIOSITY TERROIR In addition to bringing varietal In 1939, David S. Taub, Produced in the Alta Luna launched in July expressions from the Dolimiti, The Alta Luna vineyards are "�ℎ� ���ℎ�� �� ����� Dolomiti IGT, Alta Luna, 2011. It is an exciting Phases is a fusion of Merlot and Located in the foothills of the ������,” was born. meaning High Moon, range from the range from native varieties Lagrein and Adige Valley, bordering Trentino Beyond introducing Pinot has a sleek and modern the Cavit winery in Teroldego. Much like the and Alto Adige, in the Dolomiti Grigio to America, his package, featuring a die- Trentino, Italy with varying lunar phases have their IGT. The high altitude vineyards specific characteristics, each have glacial-alluvial soils, which quest was to showcase cut delineation of the Anselmo Martini as head varietal in Phases brings a Italy’s finest wines. He Dolomite Alps, creating winemaker. The vineyards particular quality to this are rich in texture and well- dedicated 4 decades of and eye-catching design for these wines are enticing red blend. Teroldego drained. The climate is typical of his life pursuing his while rooted in the Cavit carefully tended by the brings structure and notes of northern Trentino where warm dream with world- tradition. The winery families in the Cavit local raspberries. Notes of currents, temperatures in the summer renown winemakers to practices sustainable cooperative, each of cherries and red fruit are help in the development of ripe bring Pinot Grigio and farming with renewable which takes meticulous particularly intense in Lagrein concentrated fruit and elegant care of the vineyards and which also imparts balance and other indigenous energy and conservation elegance.