Saline-Water Resources of Texas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Baylor Geological Studies
BAYLORGEOLOGICA L STUDIES PAUL N. DOLLIVER Creative thinking is more important than elaborate FRANK PH.D. PROFESSOR OF GEOLOGY BAYLOR UNIVERSITY 1929-1934 Objectives of Geological Training at Baylor The training of a geologist in a university covers but a few years; his education continues throughout his active life. The purposes of train ing geologists at Baylor University are to provide a sound basis of understanding and to foster a truly geological point of view, both of which are essential for continued professional growth. The staff considers geology to be unique among sciences since it is primarily a field science. All geologic research in cluding that done in laboratories must be firmly supported by field observations. The student is encouraged to develop an inquiring ob jective attitude and to examine critically all geological concepts and principles. The development of a mature and professional attitude toward geology and geological research is a principal concern of the department. Frontis. Sunset over the Canadian River from near the abandoned settlement of Old Tascosa, Texas. The rampart-like cliffs on the horizon first inspired the name "Llano Estacado" (Palisaded Plain) among Coronado's men. THE BAYLOR UNIVERSITY PRESS WACO, TEXAS BAYLOR GEOLOGICAL STUDIES BULLETIN NO. 42 Cenozoic Evolution of the Canadian River Basin Paul N. DoUiver BAYLOR UNIVERSITY Department of Geology Waco, Texas Spring 1984 Baylor Geological Studies EDITORIAL STAFF Jean M. Spencer Jenness, M.S., Editor environmental and medical geology O. T. Ph.D., Advisor, Cartographic Editor what have you Peter M. Allen, Ph.D. urban and environmental geology, hydrology Harold H. Beaver, Ph.D. -

University of Texas Bulletin No
University of Texas Bulletin No. 2607: February IS, 1926 THE SAN ANGELO FORMATION THE GEOLOGY OF FOARD COUNTY J. W. BEEDE AND D. D. CHRISTNER BUREAU OF ECONOMIC GEOLOGY J. A. Udden, Director E. H. Sellards, Associate Director PUBLISHED BY THE UNIVERSITY OF TEXAS AUSTIN Publications of the University of Texas Publications Committee : Frederic Duncalf C. T. McCormick D. G. Cooke E. K.McGinnis J. L.Henderson H. J. MULLER E. J. Mathews Hal G Weaver The University publishes bulletins four times a month, so numbered that the first two digits of the number show the year of issue, the last two the position in the yearly series. (For example, No. 2201 is the first bulletin of the year 1922.) These comprise the official publications of the University, publications on humanistic and scientific sub- jects, bulletins prepared by the Divisionof Extension, by the Bureau of Economic Geology, and other bulletins of general educational interest. With the exception of special num- bers, any bulletin willbe sent to a citizen of Texas free on request. Allcommunications about University publications should be addressed to University Publications, University of Texas, Austin. oiSi§ste> UNIVERSITY OP TEXAS PRESS. AUSTIN University of Texas Bulletin No. 2607: February 15, 1926 THE SAN ANGELO FORMATION THE GEOLOGY OF FOARD COUNTY J. W. BEEDE AND D. D. CHRISTNER BUREAU OF ECONOMIC GEOLOGY J. A. Udden, Director E. H. Sellards, Associate Director PUBLISHED BYTHE UNIVERSITYFOUR TIMES AMONTH,AND ENTERED AS SECOND-CLASS MATTER AT THE POSTOFFICE AT AUSTIN,TEXAS, UNDER THEACT OF AUGUST 24, 1912 The benefits of education and of useful knowledge, generally diffused through a community, are essential to the preservation of a free govern- ment. -

Txu-Oclc-2601092.Pdf
BUREAU OF ECONOMIC GEOLOGY The University of Texas Austin, Texas 78712 l'eter T. Flawn, Director Report of Investigations—No. 51 Relation of Ogallala Formation to the Southern High Plains in Texas By John C. Frye and A. Byron Leonard March 1964 Contents Page Abstract 5 Introduction 5 Regional physiography 8 The Ogallala Formation and the High Plains surface 10 Fossil seeds of the Ogallala Formation 11 Pleistocene dissection of the High Plains margin 14 Abandoned Panther Valley 15 Lake Lomax 17 Fossil molluscan assemblages 18 Conclusions 20 References 20 Index . 24 - Illustrations Figures Page Index of central-western Texas location 1. map showing of profiles and fossil localities . 7 2. Regional profiles in central-western Texas 9 abandoned Panther the 3. Profiles across Lake Lomax and Valley in of and Glasscock counties - 16 Big Spring area Howard, Martin, Plate I. Field views 22 Relation of Ogallala Formation to the Southern High Plains in Texas JOHN C. FRYE 1 and A. BYRON LEONARD^ ABSTRACT Studies along the southern and south- Pliocene and Pleistocene valley is de- scribed of Edwards Plateau eastern borders of the High Plains have across a prong the of outliers of south of and the drainage of demonstrated presence Big Spring, Lake Lomax is de- fossiliferous Ogallala Formation in Borden the late Pleistocene in and Scurry counties and have documented termined to have occurred pre-Bradyan Wisconsinan lime. A the occurrence of Pliocene deposition as far meaningful physio- be- The limit of cannot be drawn southeast as Sterling County. graphic boundary southern limits of the characteristic Ash Hollow seed floras is ex- tween the High Plains and the Edwards Plateau. -

1 Running Head: SEQUENCE STRATIGRAPHY of TEXAS
Running Head: SEQUENCE STRATIGRAPHY OF TEXAS MIDDLE PERMIAN PLATFORM CARBONATES OUTCROP-BASED CHARACTERiZATION OF LEONARDIAN PLATFORM CARBONATE IN WEST TEXAS: IMPLICATIONS FOR SEQUENCE STRATIGRAPHIC STYLES IN TRANSITIONAL ICEHOUSE-GREENHOUSE SETTINGS Stephen C. Ruppel, W. Bruce Ward1, and Eduardo E. Ariza Bureau of Economic Geology The University of Texas at Austin 1 Current address: Earthworks LLC, P.O. Box 178, Newtown, CT 06470-0178 1 ABSTRACT The Sierra Diablo Mountains of West Texas contain world class exposures of lower and middle Permian platform carbonates. As such these outcrops offer key insights into the products of carbonate deposition in the transitional icehouse/greenhouse setting of the early-mid Permian that are available in few other places in the world. They also afford an excellent basis for examing how styles of facies and sequence development vary between platform tops and platform margins. Using outcrop data and observations from over 2 mi (3 km) of continuous exposure, we collected detailed data on the facies composition and architecture of high frequency (cycle-scale) and intermediate frequency (high frequency sequence scale) successions within the Leonardian. We used these data to define facies stacking patterns along depositional dip across the platform in both low and high accommodation settings and to document how these patterns vary systematically between and within sequences . These data not only provide a basis for interpreting similar Leonardian platform successions from less well constrained outcrop and subsurface data sets but also point out some important caveats that should be considered serve as an important model for understanding depositional processes during the is part of the Permian worldwide. -

2020 Draft Basin Highlights Report an Overview of Water Quality Issues Throughout the Canadian and Red River Basins
2020 DRAFT BASIN HIGHLIGHTS REPORT AN OVERVIEW OF WATER QUALITY ISSUES THROUGHOUT THE CANADIAN AND RED RIVER BASINS The preparation of this report was financed through and in cooperation with the Texas Commission on Environmental Quality North Fork Red River at FM 2473 2020 Canadian and Red River Basins Highlights Report ~ Page 2020 Canadian and Red River Basins Highlights Report ~ Page 2 Lake Texoma at US 377 Bridge TABLE OF CONTENTS CANADIAN AND RED RIVER BASIN VICINITY MAP 4 INTRODUCTION 5 Public Involvement Basin Advisory Committee Meeting 6 Coordinated Monitoring Meeting 7 Zebra Mussels Origin, Transportation, Impact, Texas Bound, Current Populations, and Studies 8 Texas Legislation Action 9 CANADIAN AND RED RIVER BASINS WATER QUALITY OVERVIEW AND HIGHLIGHTS Canadian and Red River Basins Water Quality Overview, 2018 Texas IR Overview 10 TABLES Canadian River Basin 2018 Texas IR Impairment Listing 11 Red River Basin 2018 Texas IR Impairment Listing 12 Water Quality Monitoring Field Parameters, Conventional Laboratory Parameters Red River Authority Environmental Services Laboratory Environmental Services Division 15 2020 Canadian and Red River Basins Highlights Report ~ Page 3 2020 Canadian and Red River Basins Highlights Report ~ Page 4 INTRODUCTION In 1991, the Texas Legislature enacted the Texas Clean Rivers Act (Senate Bill 818) in order to assess water quality for each river basin in the state. From this, the Clean Rivers Program (CRP) was created and has become one of the most successful cooperative efforts between federal, state, and local agen- cies and the citizens of the State of Texas. It is implemented by the Texas Commission on Environ- mental Quality (TCEQ) through local partner agencies to achieve the CRP’s primary goal of maintain- ing and improving the water quality in each river basin. -

RFP No. 212F for Endangered Species Research Projects for the Prairie Chub
1 RFP No. 212f for Endangered Species Research Projects for the Prairie Chub Final Report Contributing authors: David S. Ruppel, V. Alex Sotola, Ozlem Ablak Gurbuz, Noland H. Martin, and Timothy H. Bonner Addresses: Department of Biology, Texas State University, San Marcos, Texas 78666 (DSR, VAS, NHM, THB) Kirkkonaklar Anatolian High School, Turkish Ministry of Education, Ankara, Turkey (OAG) Principal investigators: Timothy H. Bonner and Noland H. Martin Email: [email protected], [email protected] Date: July 31, 2017 Style: American Fisheries Society Funding sources: Texas Comptroller of Public Accounts, Turkish Ministry of Education- Visiting Scholar Program (OAG) Summary Four hundred mesohabitats were sampled from 36 sites and 20 reaches within the upper Red River drainage from September 2015 through September 2016. Fishes (N = 36,211) taken from the mesohabitats represented 14 families and 49 species with the most abundant species consisting of Red Shiner Cyprinella lutrensis, Red River Shiner Notropis bairdi, Plains Minnow Hybognathus placitus, and Western Mosquitofish Gambusia affinis. Red River Pupfish Cyprinodon rubrofluviatilis (a species of greatest conservation need, SGCN) and Plains Killifish Fundulus zebrinus were more abundant within prairie streams (e.g., swift and shallow runs with sand and silt substrates) with high specific conductance. Red River Shiner (SGCN), Prairie Chub Macrhybopsis australis (SGCN), and Plains Minnow were more abundant within prairie 2 streams with lower specific conductance. The remaining 44 species of fishes were more abundant in non-prairie stream habitats with shallow to deep waters, which were more common in eastern tributaries of the upper Red River drainage and Red River mainstem. Prairie Chubs comprised 1.3% of the overall fish community and were most abundant in Pease River and Wichita River. -

Classified Stream Segments and Assessments Units Covered by Hb 4146
CLASSIFIED STREAM SEGMENTS AND ASSESSMENTS UNITS COVERED BY HB 4146 SEG ID River Basin Description Met criteria 0216 Red Wichita River Below Lake Kemp Dam 96.43% 0222 Red Salt Fork Red River 95.24% 0224 Red North Fork Red River 90.91% 1250 Brazos South Fork San Gabriel River 93.75% 1251 Brazos North Fork San Gabriel River 93.55% 1257 Brazos Brazos River Below Lake Whitney 90.63% 1415 Colorado Llano River 94.39% 1424 Colorado Middle Concho/South Concho River 95.24% 1427 Colorado Onion Creek 93.43% 1430 Colorado Barton Creek 98.25% 1806 Guadalupe Guadalupe River Above Canyon Lake 96.37% 1809 Guadalupe Lower Blanco River 95.83% 1811 Guadalupe Comal River 98.90% 1812 Guadalupe Guadalupe River Below Canyon Dam 96.98% 1813 Guadalupe Upper Blanco River 95.45% 1815 Guadalupe Cypress Creek 99.19% 1816 Guadalupe Johnson Creek 97.30% AU ID River Basin Description Met criteria 1817 Guadalupe North Fork Guadalupe River 100.00% 1414_01 Colorado Pedernales River 93.10% 1818 Guadalupe South Fork Guadalupe River 97.30% 1414_03 Colorado Pedernales River 91.38% 1905 San Antonio Medina River Above Medina Lake 100.00% 1416_05 Colorado San Saba River 100.00% 2111 Nueces Upper Sabinal River 100.00% Colorado River Below Lady Bird Lake 2112 Nueces Upper Nueces River 95.96% 1428_03 Colorado (formally Town Lake) 91.67% 2113 Nueces Upper Frio River 100.00% Medina River Below Medina 1903_04 San Antonio Diversion Lake 90.00% 2114 Nueces Hondo Creek 93.48% Medina River Below Medina 2115 Nueces Seco Creek 95.65% 1903_05 San Antonio Diversion Lake 96.84% 2309 Rio Grande Devils River 96.67% 1908_02 San Antonio Upper Cibolo Creek 97.67% 2310 Rio Grande Lower Pecos River 93.88% 2304_10 Rio Grande Rio Grande Below Amistad Reservoir 95.95% 2313 Rio Grande San Felipe Creek 95.45% 2311_01 Rio Grande Upper Pecos River 92.86% A detailed map of covered segments and assessment units can be found on the TCEQ website https://www.tceq.texas.gov/gis/nonpoint-source-project-viewer . -

Theuniversityoftexasbul
THEUNIVERSITYOFTEXASBULLETIN No. 3027: July 15, 1930 THE GEOLOGY OF STONEWALL COUNTY, TEXAS By L. T. PATTON Bureau of Economic Geology J. A. Udden, Director £. H. Sellards, Associate Director PUBLISHED BY TOE UNIVERSITY OF TEXAS AUSTIN Publications of The University of Texas Publications Committees GENERAL: Frederic Duncalf Mrs.F. A. Perry J. F.Dobie C. H. Slover J. L.Henderson G. W. Stumberg H. J.Muller A.P. Winston official E. J. Mathews Killis Campbell C. F. Arrowood C.D.Simmons E. C.H.Bantel Bryant Smith The University publishes bulletins four times a month, so numbered that the first two digits of the number show the year of issue and the last two the position in the yearly series. (For example, No. 3001is the first bulletin of the year 1930.) These bulletins comprise the official publica- tions of the University, publications on humanistic and scientific subjects, and bulletins issued from time to time by various divisions of the University. The following bureaus and divisions distribute bulletins issued by them; communications concerning bulletins in these fields should be addressed to TheUniversity of Texas,Austin,Texas,care of the bureau or division issuing the bulletin: Bureau of Business Research, Bureau of Economic Geology, Bureau of Engineering Research, Interscholastic League Bureau, andDivision of Extension. Communications concerning all other publications of the University should be addressed to University Publications,TheUniversity of Texas,Austin. Additionalcopies of this publicationmaybeprocuredfrom the Bureau of Economic Geology, The University of Texas, Austin, Texas THE UNIVERSITY OFTEXAS PREM, AUSTUI THEUNIVERSITYOF TEXASBULLETIN No. 3027: July 15, 1930 THE GEOLOGY OF STONEWALL COUNTY, TEXAS By L. -

United States Department of the Interior National Park Service Land
United States Department of the Interior National Park Service Land & Water Conservation Fund --- Detailed Listing of Grants Grouped by County --- Today's Date: 11/20/2008 Page: 1 Texas - 48 Grant ID & Type Grant Element Title Grant Sponsor Amount Status Date Exp. Date Cong. Element Approved District ANDERSON 396 - XXX D PALESTINE PICNIC AND CAMPING PARK CITY OF PALESTINE $136,086.77 C 8/23/1976 3/1/1979 2 719 - XXX D COMMUNITY FOREST PARK CITY OF PALESTINE $275,500.00 C 8/23/1979 8/31/1985 2 ANDERSON County Total: $411,586.77 County Count: 2 ANDREWS 931 - XXX D ANDREWS MUNICIPAL POOL CITY OF ANDREWS $237,711.00 C 12/6/1984 12/1/1989 19 ANDREWS County Total: $237,711.00 County Count: 1 ANGELINA 19 - XXX C DIBOLL CITY PARK CITY OF DIBOLL $174,500.00 C 10/7/1967 10/1/1971 2 215 - XXX A COUSINS LAND PARK CITY OF LUFKIN $113,406.73 C 8/4/1972 6/1/1973 2 297 - XXX D LUFKIN PARKS IMPROVEMENTS CITY OF LUFKIN $49,945.00 C 11/29/1973 1/1/1977 2 512 - XXX D MORRIS FRANK PARK CITY OF LUFKIN $236,249.00 C 5/20/1977 1/1/1980 2 669 - XXX D OLD ORCHARD PARK CITY OF DIBOLL $235,066.00 C 12/5/1978 12/15/1983 2 770 - XXX D LUFKIN TENNIS IMPROVEMENTS CITY OF LUFKIN $51,211.42 C 6/30/1980 6/1/1985 2 879 - XXX D HUNTINGTON CITY PARK CITY OF HUNTINGTON $35,313.56 C 9/26/1983 9/1/1988 2 ANGELINA County Total: $895,691.71 County Count: 7 United States Department of the Interior National Park Service Land & Water Conservation Fund --- Detailed Listing of Grants Grouped by County --- Today's Date: 11/20/2008 Page: 2 Texas - 48 Grant ID & Type Grant Element Title Grant Sponsor Amount Status Date Exp. -
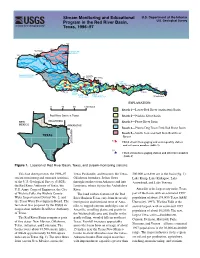
Stream Monitoring and Educational Program in the Red River Basin
Stream Monitoring and Educational U.S. Department of the Interior Program in the Red River Basin, U.S. Geological Survey Texas, 1996–97 100 o 101 o 5 AMARILLO NORTH FORK 102 o RED RIVER 103 o A S LT 35o F ORK RED R IV ER 1 4 2 PRAIRIE DOG TOWN PEASE 3 99 o WICHITA FORK RED RIVER 7 FALLS CHARLIE 6 RIVE R o o 34 W 8 98 9 I R o LAKE CHIT 21 ED 97 A . TEXOMA o VE o 10 11 R 25 96 RI R 95 16 19 18 20 DENISON 17 28 14 15 23 24 27 29 22 26 30 12,13 LAKE PARIS KEMP LAKE LAKE KICKAPOO ARROWHEAD TEXARKANA EXPLANATION 0 40 80 120 MILES Reach 1—Lower Red River (mainstem) Basin Red River Basin in Texas Reach 2—Wichita River Basin NEW OKLAHOMA Reach 3—Pease River Basin MEXICO ARKANSAS Reach 4—Prairie Dog Town Fork Red River Basin Reach 5—North Fork and Salt Fork Red River TEXAS Basins 12 LOUISIANA USGS streamflow-gaging and water-quality station and reference number (table 1) 22 USGS streamflow-gaging station and reference number (table 1) Figure 1. Location of Red River Basin, Texas, and stream-monitoring stations. This fact sheet presents the 1996–97 Texas Panhandle, and becomes the Texas- 200,000 acre-feet are in the basin (fig. 1): stream monitoring and outreach activities Oklahoma boundary. It then flows Lake Kemp, Lake Kickapoo, Lake of the U.S. Geological Survey (USGS), through southwestern Arkansas and into Arrowhead, and Lake Texoma. -

National Register Nomination File
NPS Form 10-900 OMB No. 1024-0018 United States Department of the Interior National Park Service National Register of Historic Places Registration Form 1. Name of Property Historic Name: SH 203 (Old SH 52) Bridge at Salt Fork of the Red River Other name/site number: State Highway 52 Bridge at Salt Fork of the Red River Name of related multiple property listing: Historic Road Infrastructure of Texas 2. Location Street & number: Texas State Highway 203 at Salt Fork of the Red River City or town: Wellington State: Texas County: Collingsworth Not for publication: Vicinity: 3. State/Federal Agency Certification As the designated authority under the National Historic Preservation Act, as amended, I hereby certify that this nomination request for determination of eligibility meets the documentation standards for registering properties in the National Register of Historic Places and meets the procedural and professional requirements set forth in 36 CFR Part 60. In my opinion, the property meets does not meet the National Register criteria. I recommend that this property be considered significant at the following levels of significance: national statewide local Applicable National Register Criteria: A B C D State Historic Preservation Officer ___________________________ Signature of certifying official / Title Date Texas Historical Commission State or Federal agency / bureau or Tribal Government In my opinion, the property meets does not meet the National Register criteria. _______________________________________________________________________ ___________________________ Signature of commenting or other official Date ____________________________________________________________ State or Federal agency / bureau or Tribal Government 4. National Park Service Certification I hereby certify that the property is: ___ entered in the National Register ___ determined eligible for the National Register ___ determined not eligible for the National Register. -

Index of Surface Water Stations in Texas
1 UNITED STATES DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY I AUSTIN, TEXAS INDEX OF SURFACE WATER STATIONS IN TEXAS Operated by the Water Resources Division of the Geological Survey in cooperation with State and Federal Agencies Gaging Station 08065000. Trinity River near Oakwood , October 1970 UNITED STATES DEPARTMENT OF THE INTERIOR Geological Survey - Water Resources Division INDEX OF SURFACE WATER STATIONS IN TEXAS OCTOBER 1970 Copies of this report may be obtained from District Chief. Water Resources Division U.S. Geological Survey Federal Building Austin. Texas 78701 1970 CONTENTS Page Introduction ............................... ................•.......•...•..... Location of offices .........................................•..•.......... Description of stations................................................... 2 Definition of tenns........... • . 2 ILLUSTRATIONS Location of active gaging stations in Texas, October 1970 .•.•.•.••..•••••..•.. 1n pocket TABLES Table 1. Streamflow, quality, and reservoir-content stations •.•.•... ~........ 3 2. Low-fla.o~ partial-record stations.................................... 18 3. Crest-stage partial-record stations................................. 22 4. Miscellaneous sites................................................. 27 5. Tide-level stations........................ ........................ 28 ii INDEX OF SURFACE WATER STATIONS IN TEXAS OCTOBER 1970 The U.S. Geological Survey's investigations of the water resources of Texas are con ducted in cooperation with the Texas Water Development