Araneae: Actinopodidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Miranda ZA 2018.Pdf
Zoologischer Anzeiger 273 (2018) 33–55 Contents lists available at ScienceDirect Zoologischer Anzeiger jou rnal homepage: www.elsevier.com/locate/jcz Review of Trichodamon Mello-Leitão 1935 and phylogenetic ଝ placement of the genus in Phrynichidae (Arachnida, Amblypygi) a,b,c,∗ a Gustavo Silva de Miranda , Adriano Brilhante Kury , a,d Alessandro Ponce de Leão Giupponi a Laboratório de Aracnologia, Museu Nacional do Rio de Janeiro, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista s/n, São Cristóvão, Rio de Janeiro-RJ, CEP 20940-040, Brazil b Entomology Department, National Museum of Natural History, Smithsonian Institution, 10th St. & Constitution Ave NW, Washington, DC, 20560, USA c Center for Macroecology, Evolution and Climate, Natural History Museum of Denmark (Zoological Museum), University of Copenhagen, Universitetsparken 15, 2100, Copenhagen, Denmark d Servic¸ o de Referência Nacional em Vetores das Riquetsioses (LIRN), Colec¸ ão de Artrópodes Vetores Ápteros de Importância em Saúde das Comunidades (CAVAISC), IOC-FIOCRUZ, Manguinhos, 21040360, Rio de Janeiro, RJ, Brazil a r t i c l e i n f o a b s t r a c t Article history: Amblypygi Thorell, 1883 has five families, of which Phrynichidae is one of the most diverse and with a Received 18 October 2017 wide geographic distribution. The genera of this family inhabit mostly Africa, India and Southeast Asia, Received in revised form 27 February 2018 with one genus known from the Neotropics, Trichodamon Mello-Leitão, 1935. Trichodamon has two valid Accepted 28 February 2018 species, T. princeps Mello-Leitão, 1935 and T. froesi Mello-Leitão, 1940 which are found in Brazil, in the Available online 10 March 2018 states of Bahia, Goiás, Minas Gerais and Rio Grande do Norte. -
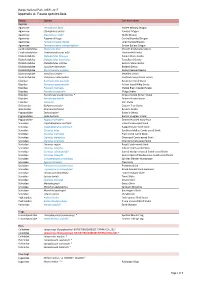
Report-Mungo National Park-Appendix A
Mungo National Park, NSW, 2017 Appendix A: Fauna species lists Family Species Common name Reptiles Agamidae Ctenophorus fordi Mallee Military Dragon Agamidae Ctenophorus pictus Painted Dragon Agamidae Diporiphora nobbi Nobbi Dragon Agamidae Pogona vitticeps Central Bearded Dragon Agamidae Tympanocryptis lineata Lined Earless Dragon Agamidae Tympanocryptis tetraporophora Eyrean Earless Dragon Carphodactylidae Nephrurus levis Smooth Knob-tailed Gecko Carphodactylidae Underwoodisaurus milii Thick-tailed Gecko Diplodactylidae Diplodactylus furcosus Ranges Stone Gecko Diplodactylidae Diplodactylus tessellatus Tessellated Gecko Diplodactylidae Diplodactylus vittatus Eastern Stone Gecko Diplodactylidae Lucasium damaeum Beaded Gecko Diplodactylidae Rhynchoedura ormsbyi Eastern Beaked Gecko Diplodactylidae Strophurus elderi ~ Jewelled Gecko Diplodactylidae Strophurus intermedius Southern Spiny-tailed Gecko Elapidae Brachyurophis australis Australian Coral Snake Elapidae Demansia psammophis Yellow-faced Whip Snake Elapidae Parasuta nigriceps Mallee Black-headed Snake Elapidae Pseudechis australis Mulga Snake Elapidae Pseudonaja aspidorhyncha * Strap-snouted Brown Snake Elapidae Pseudonaja textilis Eastern Brown Snake Elapidae Suta suta Curl Snake Gekkonidae Gehyra versicolor Eastern Tree Gecko Gekkonidae Heteronotia binoei Bynoe's Gecko Pygopodidae Delma butleri Butler's Delma Pygopodidae Lialis burtonis Burton's Legless Lizard Pygopodidae Pygopus schraderi Eastern Hooded Scaly-foot Scincidae Cryptoblepharus australis Inland Snake-eyed Skink Scincidae -

Rossi Gf Me Rcla Par.Pdf (1.346Mb)
RESSALVA Atendendo solicitação da autora, o texto completo desta dissertação será disponibilizado somente a partir de 28/02/2021. UNIVERSIDADE ESTADUAL PAULISTA “JÚLIO DE MESQUITA FILHO” Instituto de Biociências – Rio Claro Departamento de Zoologia Giullia de Freitas Rossi Taxonomia e biogeografia de aranhas cavernícolas da infraordem Mygalomorphae RIO CLARO – SP Abril/2019 Giullia de Freitas Rossi Taxonomia e biogeografia de aranhas cavernícolas da infraordem Mygalomorphae Dissertação apresentada ao Departamento de Zoologia do Instituto de Biociências de Rio Claro, como requisito para conclusão de Mestrado do Programa de Pós-Graduação em Zoologia. Orientador: Prof. Dr. José Paulo Leite Guadanucci RIO CLARO – SP Abril/2019 Rossi, Giullia de Freitas R832t Taxonomia e biogeografia de aranhas cavernícolas da infraordem Mygalomorphae / Giullia de Freitas Rossi. -- Rio Claro, 2019 348 f. : il., tabs., fotos, mapas Dissertação (mestrado) - Universidade Estadual Paulista (Unesp), Instituto de Biociências, Rio Claro Orientador: José Paulo Leite Guadanucci 1. Aracnídeo. 2. Ordem Araneae. 3. Sistemática. I. Título. Sistema de geração automática de fichas catalográficas da Unesp. Biblioteca do Instituto de Biociências, Rio Claro. Dados fornecidos pelo autor(a). Essa ficha não pode ser modificada. Dedico este trabalho à minha família. AGRADECIMENTOS Agradeço ao meus pais, Érica e José Leandro, ao meu irmão Pedro, minha tia Jerusa e minha avó Beth pelo apoio emocional não só nesses dois anos de mestrado, mas durante toda a minha vida. À José Paulo Leite Guadanucci, que aceitou ser meu orientador, confiou em mim e ensinou tudo o que sei sobre Mygalomorphae. Ao meu grande amigo Roberto Marono, pelos anos de estágio e companheirismo na UNESP Bauru, onde me ensinou sobre aranhas, e ao incentivo em ir adiante. -

Araneae: Mygalomorphae: Actinopodidae: Missulena) from the Pilbara Region, Western Australia
Zootaxa 3637 (5): 521–540 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3637.5.2 http://zoobank.org/urn:lsid:zoobank.org:pub:447D8DF5-F922-4B3A-AC43-A85225E56C57 New species of Mouse Spiders (Araneae: Mygalomorphae: Actinopodidae: Missulena) from the Pilbara region, Western Australia DANILO HARMS1, 2, 3, 4 & VOLKER W. FRAMENAU1, 2, 3 1 School of Animal Biology, The University of Western Australia, 35 Stirling Highway, Crawley, Western Australia 6009, Australia. 2 Department of Terrestrial Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, Western Australia 6986, Australia. 3 Phoenix Environmental Sciences Pty Ltd, 1/511 Wanneroo Road, Balcatta, Western Australia 6021, Australia. 4 Corresponding author. E-mail: [email protected] Abstract Two new species of Mouse Spiders, genus Missulena, from the Pilbara region in Western Australia are described based on morphological features of males. Missulena faulderi sp. nov. and Missulena langlandsi sp. nov. are currently known from a small area in the southern Pilbara only. Mitochondrial cytochrome c oxidase subunit I (COI) sequence divergence failed in clearly delimiting species in Missulena, but provided a useful, independent line of evidence for taxonomic work in addition to morphology. Key words: taxonomy, systematics, barcoding, mitochondrial DNA, short-range endemism, Actinopus, Plesiolena Introduction The Actinopodidae Simon, 1892 is a small family of mygalomorph spiders with a Gondwanan distribution that includes three genera: Actinopus Perty, 1833 (27 species), Missulena Walckenaer, 1805 (11 species) and Plesiolena Goloboff & Platnick, 1987 (two species). Actinopus and Plesiolena are known only from South and Central America (Platnick 2012). -

Australian Funnel-Web Spiders Evolved Human-Lethal Δ-Hexatoxins for Defense Against Vertebrate Predators
Australian funnel-web spiders evolved human-lethal δ-hexatoxins for defense against vertebrate predators Volker Herziga,b,1,2, Kartik Sunagarc,1, David T. R. Wilsond,1, Sandy S. Pinedaa,e,1, Mathilde R. Israela, Sebastien Dutertref, Brianna Sollod McFarlandg, Eivind A. B. Undheima,h,i, Wayne C. Hodgsonj, Paul F. Alewooda, Richard J. Lewisa, Frank Bosmansk, Irina Vettera,l, Glenn F. Kinga,2, and Bryan G. Frym,2 aInstitute for Molecular Bioscience, The University of Queensland, St Lucia, QLD 4072, Australia; bGeneCology Research Centre, School of Science and Engineering, University of the Sunshine Coast, Sippy Downs, QLD 4556, Australia; cEvolutionary Venomics Lab, Centre for Ecological Sciences, Indian Institute of Science, Bangalore 560012, India; dCentre for Molecular Therapeutics, Australian Institute of Tropical Health and Medicine, James Cook University, Smithfield, QLD 4878, Australia; eBrain and Mind Centre, University of Sydney, Camperdown, NSW 2052, Australia; fInstitut des Biomolécules Max Mousseron, UMR 5247, Université Montpellier, CNRS, 34095 Montpellier Cedex 5, France; gSollod Scientific Analysis, Timnath, CO 80547; hCentre for Advanced Imaging, The University of Queensland, St Lucia, QLD 4072, Australia; iCentre for Ecology and Evolutionary Synthesis, Department of Biosciences, University of Oslo, 0316 Oslo, Norway; jMonash Venom Group, Department of Pharmacology, Monash University, Clayton, VIC 3800, Australia; kBasic and Applied Medical Sciences Department, Faculty of Medicine, Ghent University, 9000 Ghent, Belgium; lSchool -

Antivenoms for the Treatment of Spider Envenomation
† Antivenoms for the Treatment of Spider Envenomation Graham M. Nicholson1,* and Andis Graudins1,2 1Neurotoxin Research Group, Department of Heath Sciences, University of Technology, Sydney, New South Wales, Australia 2Departments of Emergency Medicine and Clinical Toxicology, Westmead Hospital, Westmead, New South Wales, Australia *Correspondence: Graham M. Nicholson, Ph.D., Director, Neurotoxin Research Group, Department of Heath Sciences, University of Technology, Sydney, P.O. Box 123, Broadway, NSW, 2007, Australia; Fax: 61-2-9514-2228; E-mail: Graham. [email protected]. † This review is dedicated to the memory of Dr. Struan Sutherland who’s pioneering work on the development of a funnel-web spider antivenom and pressure immobilisation first aid technique for the treatment of funnel-web spider and Australian snake bites will remain a long standing and life-saving legacy for the Australian community. ABSTRACT There are several groups of medically important araneomorph and mygalomorph spiders responsible for serious systemic envenomation. These include spiders from the genus Latrodectus (family Theridiidae), Phoneutria (family Ctenidae) and the subfamily Atracinae (genera Atrax and Hadronyche). The venom of these spiders contains potent neurotoxins that cause excessive neurotransmitter release via vesicle exocytosis or modulation of voltage-gated sodium channels. In addition, spiders of the genus Loxosceles (family Loxoscelidae) are responsible for significant local reactions resulting in necrotic cutaneous lesions. This results from sphingomyelinase D activity and possibly other compounds. A number of antivenoms are currently available to treat envenomation resulting from the bite of these spiders. Particularly efficacious antivenoms are available for Latrodectus and Atrax/Hadronyche species, with extensive cross-reactivity within each genera. -

C:\Documents and Settings\Administrator\My Files
ISSN - 0797 - 6828 ANALES MUSEO NACIONAL DE HISTORIA NATURAL Y ANTROPOLOGIA (2ª Serie) Vol. 10, No 5 2003 Diversidad de la Biota Uruguaya ARANEAE ROBERTO M. CAPOCASALE & ANDREA PEREIRA * ABSTRACT: This paper deals with 177 Araneae species that occur in the Rep. O. del Uruguay indicated until December 31, 2001. These species are distributed in 36 families. Argyrodes altissimus (Mello-Leitão) is indicated for the first time in Uruguay. The item "Distribución" confirms the geographic departments in which some of the mentioned species were collected. The information offered was based on the specimens deposited in the Museo de Historia Natural y de Antropología and Sección Entomología, Faculty of Sciences, Collections from Uruguay and in the literature. Key words: Araneae, Uruguay, spiders taxonomy, spiders Palabras clave: Araneae, Uruguay, taxonomía de Araneae, arañas Introducción Estudiar la Biodiversidad Alfa de una región impone que se tenga un conocimiento más o menos completo de todos los táxones que integran esa región. En América del Sur, por ahora, eso es casi una utopía, si se considera la carencia cada vez mayor de sistematas y de especialistas en cada grupo zoológico. Por * Instituto de Investigaciones Biológicas Clemente Estable. Dep. Zoología Experimental. Av. Italia 3318, 11.600 Montevideo, Uruguay. E-mail: [email protected] 2 CAPOCASALE & PEREIRA: Araneae del Uruguay [10(5) supuesto, Uruguay no escapa a dicha realidad. En la República O. del Uruguay desde 1833, fecha en la cual PERTY indicó la primera especie de araña para el país (Actinopus tarsalis), hasta la década del 70 del siglo pasado, nunca antes había existido un artículo que contuviese una lista exclusiva para Uruguay que abarcara todas las especies indicadas más la mayoría de las existentes en las colecciones nacionales. -

(Mygalomorphae, Atracinae), with Implications for Venom
www.nature.com/scientificreports OPEN Phylogenomic reclassifcation of the world’s most venomous spiders (Mygalomorphae, Atracinae), with Received: 10 November 2017 Accepted: 10 January 2018 implications for venom evolution Published: xx xx xxxx Marshal Hedin1, Shahan Derkarabetian 1,2, Martín J. Ramírez3, Cor Vink4 & Jason E. Bond5 Here we show that the most venomous spiders in the world are phylogenetically misplaced. Australian atracine spiders (family Hexathelidae), including the notorious Sydney funnel-web spider Atrax robustus, produce venom peptides that can kill people. Intriguingly, eastern Australian mouse spiders (family Actinopodidae) are also medically dangerous, possessing venom peptides strikingly similar to Atrax hexatoxins. Based on the standing morphology-based classifcation, mouse spiders are hypothesized distant relatives of atracines, having diverged over 200 million years ago. Using sequence- capture phylogenomics, we instead show convincingly that hexathelids are non-monophyletic, and that atracines are sister to actinopodids. Three new mygalomorph lineages are elevated to the family level, and a revised circumscription of Hexathelidae is presented. Re-writing this phylogenetic story has major implications for how we study venom evolution in these spiders, and potentially genuine consequences for antivenom development and bite treatment research. More generally, our research provides a textbook example of the applied importance of modern phylogenomic research. Atrax robustus, the Sydney funnel-web spider, is ofen considered the world’s most venomous spider species1. Te neurotoxic bite of a male A. robustus causes a life-threatening envenomation syndrome in humans. Although antivenoms have now largely mitigated human deaths, bites remain potentially life-threatening2. Atrax is a mem- ber of a larger clade of 34 described species, the mygalomorph subfamily Atracinae, at least six of which (A. -

Araneae: Mygalomorphae: Actinopodidae: Missulena) from the Pilbara Region, Western Australia
Zootaxa 3637 (5): 521–540 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3637.5.2 http://zoobank.org/urn:lsid:zoobank.org:pub:447D8DF5-F922-4B3A-AC43-A85225E56C57 New species of Mouse Spiders (Araneae: Mygalomorphae: Actinopodidae: Missulena) from the Pilbara region, Western Australia DANILO HARMS1, 2, 3, 4 & VOLKER W. FRAMENAU1, 2, 3 1 School of Animal Biology, The University of Western Australia, 35 Stirling Highway, Crawley, Western Australia 6009, Australia. 2 Department of Terrestrial Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, Western Australia 6986, Australia. 3 Phoenix Environmental Sciences Pty Ltd, 1/511 Wanneroo Road, Balcatta, Western Australia 6021, Australia. 4 Corresponding author. E-mail: [email protected] Abstract Two new species of Mouse Spiders, genus Missulena, from the Pilbara region in Western Australia are described based on morphological features of males. Missulena faulderi sp. nov. and Missulena langlandsi sp. nov. are currently known from a small area in the southern Pilbara only. Mitochondrial cytochrome c oxidase subunit I (COI) sequence divergence failed in clearly delimiting species in Missulena, but provided a useful, independent line of evidence for taxonomic work in addition to morphology. Key words: taxonomy, systematics, barcoding, mitochondrial DNA, short-range endemism, Actinopus, Plesiolena Introduction The Actinopodidae Simon, 1892 is a small family of mygalomorph spiders with a Gondwanan distribution that includes three genera: Actinopus Perty, 1833 (27 species), Missulena Walckenaer, 1805 (11 species) and Plesiolena Goloboff & Platnick, 1987 (two species). Actinopus and Plesiolena are known only from South and Central America (Platnick 2012). -

Phylogeny and Classification of Spiders
18 FROM: Ubick, D., P. Paquin, P.E. Cushing, andV. Roth (eds). 2005. Spiders of North America: an identification manual. American Arachnological Society. 377 pages. Chapter 2 PHYLOGENY AND CLASSIFICATION OF SPIDERS Jonathan A. Coddington ARACHNIDA eyes, jumping spiders also share many other anatomical, Spiders are one of the eleven orders of the class Arach- behavioral, ecological, and physiological features. Most nida, which also includes groups such as harvestmen (Opil- important for the field arachnologist they all jump, a useful iones), ticks and mites (Acari), scorpions (Scorpiones), false bit of knowledge if you are trying to catch one. Taxonomic scorpions (Pseudoscorpiones), windscorpions (Solifugae), prediction works in reverse as well: that spider bouncing and vinegaroons (Uropygi). All arachnid orders occur in about erratically in the bushes is almost surely a salticid. North America. Arachnida today comprises approximately Another reason that scientists choose to base classifica- 640 families, 9000 genera, and 93,000 described species, but tion on phylogeny is that evolutionary history (like all his- the current estimate is that untold hundreds of thousands tory) is unique: strictly speaking, it only happened once. of new mites, substantially fewer spiders, and several thou- That means there is only one true reconstruction of evolu- sand species in the remaining orders, are still undescribed tionary history and one true phylogeny: the existing clas- (Adis & Harvey 2000, reviewed in Coddington & Colwell sification is either correct, or it is not. In practice it can be 2001, Coddington et ol. 2004). Acari (ticks and mites) are complicated to reconstruct the true phylogeny of spiders by far the most diverse, Araneae (spiders) second, and the and to know whether any given reconstruction (or classifi- remaining taxa orders of magnitude less diverse. -

BIOSYSTEMATICS of AUSTRALIAN MYGALOMORPH SPIDERS DESCRIPTION of a NEW SPECIES of MISSULENA FROM... Download 1.45 MB
Records of the Western Australian Museum 17: 355-359 (1996). Biosystematics of Australian mygalomorph spiders: description of a new species of Missulena from southwestern Australia (Araneae: Mygalomorphae: Actinopodidae) Barbara York Main Department of Zoology, University of Western Australia, Nedlands, Western Australia 6009, Australia Abstract -A new species of Missulena from west of Albany, southwestern Australia is described and notes on its biology presented. Missulena rugosum Ausserer is tentatively reinstated. INTRODUCTION occurs also in the same area. The trapdoor spider genus Missulena Abbreviations: BYM, Barbara York Main collec Walckenaer, the only Australian representative of tion (housed in the Zoology Department, Univer the Gondwanan family Actinopodidae, is sity of Western Australia); WAM, Western Austra widespread across the continent and occurs on lian Museum. some offshore islands but is absent from Tasmania. Early records of the genus in New Guinea (Rainbow 1920) are now generally discounted. The genus has long been recognised as taxonomically SYSTEMATICS difficult. It was the first indigenous mygalomorph genus named from Australia. However, in spite of Missulena torbayensis sp. novo a revision of the genus by Womersley (1943) Figures 1, 2; Table 1 recognition of nominal species is in most cases difficult due to a combination of inadequate early Material examined nineteenth century descriptions, loss of many types, absence of locality data for early specimens Holotype and the conservative morphology. Perhaps the only 0, Rutherwood Road/South Coast Highway named species which are uneqivocally recognisable (Highway 1), Torbay, Nature Reserve, Western Australia, Australia, 16 June 1983, B.Y. Main, alive are M. pruinosa Levitt-Gregg from northern Australia, M. -

Promoting Excellence of Monographs in Taxonomy
Megataxa 001 (2): 141–142 ISSN 2703-3082 (print edition) https://www.mapress.com/j/mt/ MEGATAXA Copyright © 2020 Magnolia Press Editorial ISSN 2703-3090 (online edition) https://doi.org/10.11646/megataxa.1.2.4 http://zoobank.org/urn:lsid:zoobank.org:pub:CC85771A-2195-4BDC-AB3F-2384922F208C Promoting excellence of monographs in taxonomy ZHI-QIANG ZHANG1,2 1Manaaki Whenua—Landcare Research, 231 Morrin Road, Auckland, New Zealand; �[email protected]; https://orcid.org/0000-0003-4172-0592 2Centre for Biodiversity & Biosecurity, School of Biological Sciences, University of Auckland, Auckland, New Zealand Megataxa was designed mainly for large monographic printed pages; only 7.8% of the monographs published in reviews and highly significant original papers reporting Zootaxa during 2019 and 2020 meet this requirement. major advances in taxonomy (Zhang 2020). Monographs, It is my great delight to announce the publication being comprehensive treatments of various taxa, are the of the first monograph in Megataxa on 80 species of most important works in taxonomy. They are extremely mygalomorph spiders of the genus Actinopus (Miglio et important to understanding biodiversity, but they require al. 2020). This monograph was originally submitted to so much expertise and efforts that they are rarely attempted Zootaxa and edited by Dr Robert Raven. by many taxonomists these days (Muñoz-Rodríguez et Mygalomorph spiders are unequivocally difficult al. 2019). In Zootaxa and Phytotaxa, monographs are spiders taxonomically: many are depauperate of easily operationally categorized as large papers of 60 or more recognizable characters which often show spectacular printed pages. Last year, Zootaxa published 118 such bilateral variability and serious sexual dimorphism.