Preparation of Amine Alanes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Production of Malonic Acid Through the Fermentation of Glucose
University of Pennsylvania ScholarlyCommons Department of Chemical & Biomolecular Senior Design Reports (CBE) Engineering 4-20-2018 Production of Malonic Acid through the Fermentation of Glucose Emily P. Peters University of Pennsylvania, [email protected] Gabrielle J. Schlakman University of Pennsylvania, [email protected] Elise N. Yang University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/cbe_sdr Part of the Biochemical and Biomolecular Engineering Commons Peters, Emily P.; Schlakman, Gabrielle J.; and Yang, Elise N., "Production of Malonic Acid through the Fermentation of Glucose" (2018). Senior Design Reports (CBE). 107. https://repository.upenn.edu/cbe_sdr/107 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/cbe_sdr/107 For more information, please contact [email protected]. Production of Malonic Acid through the Fermentation of Glucose Abstract The overall process to produce malonic acid has not drastically changed in the past 50 years. The current process is damaging to the environment and costly, requiring high market prices. Lygos, Inc., a lab in Berkeley, California, has published a patent describing a way to produce malonic acid through the biological fermentation of genetically modified easty cells. This proposed technology is appealing as it is both better for the environment and economically friendly. For the process discussed in this report, genetically modified Pichia Kudriavzevii yeast cells will be purchased from the Lygos lab along with the negotiation of exclusive licensing rights to the technology. The cells will be grown in fermentation vessels, while being constantly fed oxygen, glucose and fermentation media. The cells will excrete malonic acid in the 101 hour fermentation process. -

WO 2013/089962 Al 20 June 2013 (20.06.2013) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/089962 Al 20 June 2013 (20.06.2013) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every B01J 31/04 (2006.01) B01J 31/18 (2006.01) kind of national protection available): AE, AG, AL, AM, B01J 31/14 (2006.01) B01J 31/22 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US20 12/065285 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 15 November 2012 (15.1 1.2012) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (25) Filing Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (26) Publication Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 13/323,328 12 December 201 1 (12. 12.201 1) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): CHEV¬ GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, RON PHILLIPS CHEMICAL COMPANY LP UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, [US/US]; 10001 Six Pines Drive, The Woodlands, Texas TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, 77380 (US). -

PESTICIDES Criteria for a Recommended Standard
CRITERIA FOR A RECOMMENDED STANDARD OCCUPATIONAL EXPOSURE DURING THE MANUFACTURE AND FORMULATION OF PESTICIDES criteria for a recommended standard... OCCUPATIONAL EXPOSURE DURING THE MANUFACTURE AND FORMULATION OF PESTICIDES * U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service Center for Disease Control National Institute for Occupational Safety and Health July 1978 For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 DISCLAIMER Mention of company names or products does not constitute endorsement by the National Institute for Occupational Safety and Health. DHEW (NIOSH) Publication No. 78-174 PREFACE The Occupational Safety and Health Act of 1970 emphasizes the need for standards to protect the health and provide for the safety of workers occupationally exposed to an ever-increasing number of potential hazards. The National Institute for Occupational Safety and Health (NIOSH) has implemented a formal system of research, with priorities determined on the basis of specified indices, to provide relevant data from which valid criteria for effective standards can be derived. Recommended standards for occupational exposure, which are the result of this work, are based on the effects of exposure on health. The Secretary of Labor will weigh these recommendations along with other considerations, such as feasibility and means of implementation, in developing regulatory standards. Successive reports will be presented as research and epideiriologic studies are completed and as sampling and analytical methods are developed. Criteria and standards will be reviewed periodically to ensure continuing protection of workers. The contributions to this document on pesticide manufacturing and formulating industries by NIOSH staff members, the review consultants, the reviewer selected by the American Conference of Governmental Industrial Hygienists (ACGIH), other Federal agencies, and by Robert B. -

Synthesis of Insecticidal Mono- and Diacylhydrazines for Disruption of K+ Voltage-Gated Channels, Elucidation of Regiochemistry, And
Synthesis of Insecticidal Mono- and Diacylhydrazines for Disruption of K+ Voltage-Gated Channels, Elucidation of Regiochemistry, and Description of Conformational Isomerism by NMR Spectroscopy and Computation Joseph Shelby Clements II Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Chemistry Paul R. Carlier, Chair David G. I. Kingston James M. Tanko Gordon T. Yee May 2, 2017 Blacksburg, VA Keywords: Hydrazine, acylation, α-effect, regioselectivity, hydrazide, voltage- gated, (E)-/(Z)-, 19F NMR, single electron transfer (SET), rotational barrier, N-N bond rotation, rotational isomerism, helicity, transition state, dihedral angle Copyright 2017, Joseph S. Clements II Synthesis of Insecticidal Mono- and Diacylhydrazines for Disruption of K+ Voltage-Gated Channels, Elucidation of Regiochemistry, and Description of Conformational Isomerism by NMR Spectroscopy and Computation Joseph Shelby Clements II ABSTRACT Based on the success of diacyl-tert-butylhydrazines RH-5849 and RH-1266 in controlling agricultural crop pests, we endeavored to synthesize our own diacylbenzyl- and arylhydrazine derivatives for use against the malaria vector Anopheles gambiae. In the process of producing a library of compounds for assay against An. gambiae, it became clear that employing regioselective acylation techniques (in molecules that feature two nucleophilic, acyclic nitrogen atoms α to one another) would be imperative. Synthesis of the library derivatives proceeded rapidly and after topical assay, we found three compounds that were more toxic than the RH- series leads. One of the three displayed an LD50 value of half that of RH-1266, though patch clamp assay concluded that toxicity was not necessarily linked to inhibition of mosquito K+ channel Kv2.1. -

Synthesis of Novel Extremely Sterically Hindered Tertiary Alkylamines
Synthesis of Novel Extremely Sterically Hindered Tertiary Alkylamines von der Fakultät für Naturwissenschaften der Technischen Universität Chemnitz genehmigte Dissertation zur Erlangung des akademischen Grades doktor rerum naturalium (Dr. rer. nat.) vorgelegt von M.Sc. Tharallah A. Shoker geboren am 18. April 1985 in Nabi Chit, Lebanon eingereicht am 17. January 2018 Gutachter: Prof. Dr. Klaus Banert JP Dr. Evgeny Kataev Tag der Verteidigung: 16. April 2018 Die vorliegende Arbeit wurde in der Zeit von Oktober 2015 bis Oktober 2017 am Institut für Chemie an der Fakultät für Naturwissenschaften der Technischen Universität Chemnitz unter der Leitung von Prof. Dr. Klaus Banert angefertigt. 2 Bibliographic Description Synthesis of Novel Extremely Sterically Hindered Tertiary Alkylamines SHOKER, THARALLAH Technische Universität Chemnitz, Fakultät für Naturwissenschaften Dissertation, 2017, 208 pages. Abstract Three advanced methodologies for the preparation of extremely sterically hindered tertiary alkyl amines have been developed. The syntheses of 28 novel tertiary alkylamines that accommodate unusual steric hindrance are detailed. The electrophilic amination of alkyl Grignard reagents with N-chlorodialkylamines, in the presence of N,N,N′,N′-tetramethylethylenediamine (TMEDA) as a key additive, gives a variety of unprecedentedly sterically hindered tertiary alkylamines in good yields. Alternative strategy to 1-adamantyl-substituted (1-Ad) sterically hindered tertiary amines, which involved instead an SN1 reaction between 1-Ad cation with various secondary amines, is described. A complementary strategy to 1-Ad-based sterically hindered tertiary amines, which involves an iminium salt intermediate, is also reported. Salient features of the three protocols that are detailed here include unusual tolerance of steric hindrance, mild reaction conditions employed, ease of product isolation-purification, and absence of catalysts/transition metals. -

The Synthesis and Characterization of Energetic Materials from Sodium Azide
THE SYNTHESIS AND CHARACTERIZATION OF ENERGETIC MATERIALS FROM SODIUM AZIDE A Thesis Presented to The Academic Faculty By Joshua Aronson In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy in the School of Chemistry and Biochemistry Georgia Institute of Technology October 2004 THE SYNTHESIS AND CHARACTERIZATION OF ENERGETIC MATERIALS FROM SODIUM AZIDE Approved by: Charles L. Liotta, Advisor Kent Richman Charles A. Eckert Julia Kubanek David Collard November 17, 2004 ii This thesis is dedicated to M. G. iii ACKNOWLEDGEMENT I would first like to thank Dr. Liotta and Dr. Eckert for their support and encouragement. I will forever envy your enthusiasm and spirit. I would also like to extend my appreciation to everyone at American Pacific. My experiences in Utah and Las Vegas contributed a great deal to my graduate education. In addition, I want to thank the entire Liotta-Eckert research group—especially Kris and Pamela. The two of you made all of this not only bearable, but fun. Finally, I would like to thank my mom and Mary Katherine for their support. I would not have made it without you. iv TABLE OF CONTENTS Acknowledgement iv List of Figures viii List of Tables xii List of Abbreviations xiii Summary xv Chapter I Introduction 1 Chapter II A Novel Approach To The Synthesis Of Tetrazoles 5 Introduction 5 Experimental Methods 10 Materials 10 Apparatus 10 Procedures 11 Results and Discussion 14 Conclusion 37 References 38 Chapter III The Synthesis and Characterization Of Glycidyl 40 Azide Polymer Introduction 40 Experimental -

WO 2017/061959 Al 13 April 2017 (13.04.2017) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/061959 Al 13 April 2017 (13.04.2017) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A01P 7/00 (2006.01) A01N 25/28 (2006.01) kind of national protection available): AE, AG, AL, AM, A01P 7/02 (2006.01) A01N 25/04 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A01P 7/04 (2006.01) A01N 43/90 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A01P 5/00 (2006.01) A01N 53/06 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, EST, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/TR2015/000350 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 2 December 2015 (02.12.2015) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 2014/14665 4 December 2014 (04. -

2,4-Dichlorophenoxyacetic Acid Analysis of Risk to Endangered And
2,4-Dichlorophenoxyacetic Acid Analysis of Risks to Endangered and Threatened Salmon and Steelhead December 1, 2004 Shannon Borges Cara Dzubow Greg Orrick Ann Stavola Environmental Field Branch Office of Pesticide Programs Summary 2,4-D is a widely used herbicide applied to many crop sites and non-crop sites in the Pacific Northwest. Acid and amine salt forms of 2,4-D are "practically non-toxic" to “slightly toxic,” while ester forms of 2,4-D are “slightly toxic” to “highly toxic” to fish. Some forms of 2,4-D may lead to adverse acute and chronic effects. Some esters are “moderately toxic” to freshwater invertebrates, which may be sources of food for salmonids. All forms of 2,4-D that were tested had relatively high toxicity to vascular plants, which could lead to loss of cover for salmonid adults and young. Terrestrial uses of 2,4-D in the Pacific Northwest pose no direct or indirect risk to fish. 2,4-D acid and amine salts pose an indirect risk to fish when used on rice crops and for aquatic weed control. 2,4-D esters pose acute and chronic risk directly and indirectly when used for aquatic weed control. This endangered species risk assessment is developed for Federally listed Pacific salmon and steelhead. The findings of the Office of Pesticide Program’s Environmental Risk Assessment developed for non-target fish and wildlife as part of the reregistration process are applied to determine the potential risks to the 26 listed threatened and endangered Evolutionarily Significant Units (ESUs) of Pacific salmon and steelhead. -
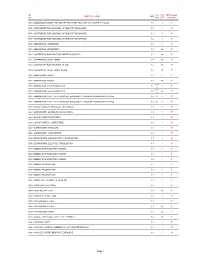
DG Classification.Pdf
UN Sub PSA MPA weight CHEMICAL NAME IMO No Risk GRP restriction 0004 AMMONIUM PICRATE, DRY OR WETTED WITH LESS THAN 10% WATER, BY MASS 1.1 1 Y* 0005 CARTRIDGES FOR WEAPONS, WITH BURSTING CHARGE 1.1 1 Y* 0006 CARTRIDGES FOR WEAPONS, WITH BURSTING CHARGE 1.1 1 Y* 0007 CARTRIDGES FOR WEAPONS, WITH BURSTING CHARGE 1.2 1 Y* 0009 AMMUNITION, INCENDIARY, 1.2 1 Y* 0010 AMMUNITION, INCENDIARY, 1.3 2A Y 0012 CARTRIDGES FOR WEAPONS, INERT PROJECTILE 1.4 2A Y 0012 CARTRIDGES, SMALL ARMS 1.4 2A Y 0014 CARTRIDGES FOR WEAPONS, BLANK 1.4 2A Y 0014 CARTRIDGES, SMALL ARMS, BLANK 1.4 2A Y 0015 AMMUNITION, SMOKE, 1.2 1 Y* 0016 AMMUNITION, SMOKE, 1.3 2A Y 6.1 / 0018 AMMUNITION, TEAR-PRODUCING, 1.2 1 Y* 8 6.1 / 0019 AMMUNITION, TEAR-PRODUCING, 1.3 2A Y 8 0020 AMMUNITION, TOXIC, WITH BURSTER, EXPELLING CHARGE OR PROPELLING CHARGE 1.2 6.1 1 Y* 0021 AMMUNITION, TOXIC, WITH BURSTER, EXPELLING CHARGE OR PROPELLING CHARGE 1.3 6.1 1 Y* 0027 BLACK POWDER, GRANULAR, OR AS MEAL 1.1 1 Y* 0027 GUNPOWDER, GRANULAR, OR AS A MEAL 1.1 1 Y* 0028 BLACK POWDER IN PELLETS 1.1 1 Y* 0028 BLACK POWDER, COMPRESSED 1.1 1 Y* 0028 GUNPOWDER IN PELLETS 1.1 1 Y* 0028 GUNPOWDER, COMPRESSED 1.1 1 Y* 0029 DETONATORS, NON-ELECTRIC, FOR BLASTING 1.1 1 Y* 0030 DETONATORS, ELECTRIC, FOR BLASTING 1.1 1 Y* 0033 BOMBS, WITH BURSTING CHARGE 1.1 1 Y* 0034 BOMBS, WITH BURSTING CHARGE 1.1 1 Y* 0035 BOMBS, WITH BURSTING CHARGE 1.2 1 Y* 0037 BOMBS, PHOTO-FLASH 1.1 1 Y* 0038 BOMBS, PHOTO-FLASH 1.1 1 Y* 0039 BOMBS, PHOTO-FLASH 1.2 1 Y* 0042 BOOSTERS, WITHOUT DETONATOR 1.1 1 Y* 0043 BURSTERS, EXPLOSIVE 1.1 -

Composition for a Resin
~™ II 1 1 III II II 1 1 IMI I III II I II 1 1 (19) J European Patent Office Office europeen des brevets (11) EP 0 936 233 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) int. CI.6: C08G 18/00, C08G 18/77, 18.08.1999 Bulletin 1999/33 C08G 59/30, G02B 1/04 (21) Application number: 99102182.5 (22) Date of filing: 04.02.1999 (84) Designated Contracting States: • Takeuchi, Motoharu, AT BE CH CY DE DK ES Fl FR GB GR IE IT LI LU c/o Mitsubishi Gas Chemical Co MCNLPTSE Tokyo (JP) Designated Extension States: • Mizuno, Katsuyuki, AL LT LV MK RO SI c/o Mitsubishi Gas Chemical Co. Tokyo (JP) (30) Priority: 10.02.1998 JP 2848198 (74) Representative: (71) Applicant: Turk, Gille, Hrabal MITSUBISHI GAS CHEMICAL COMPANY, INC. Patentanwalte - European Patent Attorneys, Chiyoda-ku, Tokyo (JP) Brucknerstrasse 20 40593 Dusseldorf (DE) (72) Inventors: • Amagai, Akikazu, c/o Mitsubishi Gas Chemical Co. Tokyo (JP) (54) Composition for a resin (57) A composition comprising a compound having one or more episulfide groups and/or epoxy groups in one molecule and a compound having one or more iso- cyanate groups and/or thioisocyanate groups in one molecule. The composition for a resin comprising a sulfur- containing compound exhibits an excellent balance between a refractivity index and an Abbe number and has a large impact resistance. CM < CO CO CM CO CO <7> O Q_ LU Printed by Xerox (UK) Business Services 2.16.7/3.6 EP 0 936 233 A2 Description BACKGROUND OF THE INVENTION 5 1 . -
![United States Patent [19] [11] Patent Number: 4,518,778 Cuscurida [45] Date of Patent: May 21, 1985](https://docslib.b-cdn.net/cover/7063/united-states-patent-19-11-patent-number-4-518-778-cuscurida-45-date-of-patent-may-21-1985-4117063.webp)
United States Patent [19] [11] Patent Number: 4,518,778 Cuscurida [45] Date of Patent: May 21, 1985
United States Patent [19] [11] Patent Number: 4,518,778 Cuscurida [45] Date of Patent: May 21, 1985 [54] POLYMER POLYOLS FROM ALKYLENE 4,089,835 5/1978 Konig et a1. ...................... .. 252/308 OXIDE ADDUCTS OF ALKANOLAMINES guscul'iga -- 222; . , , USCUrl a ............ .. [75] Inventor: Michael Cuscurida, Austin, Tex‘ 4,309,532 l/l982 Cuscurida ........ .. .. 544/398 [73] Assigneez Texaco Inc" White Plains, N_Y_ 4,374,209 2/1983 Rowlands ......................... .. 521/166 2 A . N .: 135 Primary Examiner-Donald G. Daus [ 1] pp! 0 5 ’ 99 Assistant Examiner—Cecilia Shen [22] Filed! Jlll- 14, 1983 Attorney, Agent, or Firm-Jack H. Park; Kenneth R. [51] Int. (:1.3 .................. .. c071) 241/04; CO8G 18/28; Pr‘em; Dav'd L- Mossma“ CO7C 125/06 [57] ABSTRACT [52] US. Cl. .................................. .. 544/398; 252/182; _ 521/164; 521/166; 528/73; 528/76; 560/26; A polymer polyol made by the reactlon of an alkylene 560/115; 560/158 oxide adduct of a hydroxyl-containing amine, a poly [58] Field of Search .................. .. 544/398; 528/73, 76; ether polyol of about 3000 to 8000 molecular weight 560/26, 115, 158; 252/182; 521/164, 166 and an organic polyisocyanate 1s disclosed. This poly R C_ d mer polyol may be used in the manufacture of ?exible [56] eferences "8 polyurethane foams. The polymer polyols are more US. PATENT DOCUMENTS stable than those made by other procedures. 3,294,751 12/1966 Beitchman .......................... .. 528/77 3,325,421 6/1967 Muller ............................... .. 252/308 16 Claims, N0 Drawings 4,518,778 1 2 isocyanates in a polyol solvent are U.S. -

Aldrich FT-IR Collection Edition I Supplement
Aldrich FT-IR Collection Edition I Supplement Library Listing – 7,949 spectra This library is a collection of spectra not found in the original Aldrich Collection of FT-IR Spectra Edition I. Combining this collection with the Edition I collection provides the same chemical compound coverage as the Aldrich Collection of FT- IR Spectra Edition II. The Aldrich Collection of FT-IR Spectra Edition I Supplement covers a wide variety of functional groups and was acquired using the same standards and procedures as the original library. This collection includes 7,949 spectra and comes with the Second Edition of the Aldrich Library of FT-IR spectra reference books. Aldrich FT-IR Collection Edition I Supplement Index Compound Name Index Compound Name 976 ((1S)-ENDO)-(-)-3- 4014 (+)-6-METHOXY-ALPHA- BROMOCAMPHOR, 98% METHYL-2- 2816 ((4-(1-HEPTYL-8-(3- NAPHTHALENEACETIC ACID, (OXIRANYLMETHOXY)PHENYL 98% )OCTYL) 7238 (+)-B- PHENOXY)METHYL)OXIR METHOXYDIISOPINOCAMPHEY 7537 ((R)-(+)-2,2'- LBORANE BIS(DIPHENYLPHOSPHINO)- 99 (+)-BETA-PINENE, 98% 1,1'-BINAPH)(1,5- 4524 (+)-BICUCULLINE, 98% CYCLOOCTADIENE) 3435 (+)-BOLDINE 7539 ((R)-(+)-2,2'- HYDROCHLORIDE, 99% BIS(DIPHENYLPHOSPHINO)- 282 (+)-BORNEOL, 98% 1,1'- 1182 (+)-CAMPHORCARBOXYLIC BINAPHTHYL)PALLADIUM(II) ACID, 98%, MIXTURE OF ENDO CH AND EXO 5306 ((R)-(+)-3-HYDROXY-2- 410 (+)-CHLOROMETHYL METHYLPROPYL)- TRIPHENYL- MENTHYL ETHER, 97% PHOSPHONIUM BROMIDE, 9 275 (+)-CIS-P-MENTHANE-3,8-DIOL, 7538 ((S)-(-)-2,2'- 97% BIS(DIPHENYLPHOSPHINO)- 4441 (+)-COREY LACTONE, 4- 1,1'-BINAPH)(1,5- PHENYLBENZOATE ALCOHOL,