Size and Fiber Density Controlled Synthesis Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

To Reformative Theory of Punishment”
“AN EYE FOR AN EYE WILL TURN THE WHOLE WORLD BLIND - IN SPECIAL CONTEXT TO REFORMATIVE THEORY OF PUNISHMENT” Rustam Singh Thakur1 “An eye for an eye will turn the whole world blind” – Mahatma Gandhi2, This line by Mahatma Gandhi is the thrust of the Reformative Theory of Punishment . The most recent and the most humane of all theories are based on the principle of reforming the legal offenders through individual treatment. Not looking to criminals as inhuman this theory puts forward the changing nature of the modern society where it presently looks into the fact that all other theories have failed to put forward any such stable theory, which would prevent the occurrence of further crime3. Reform in the deterrent sense implied that through being punished the offender recognized his guilt and wished to change. This theory aims at rehabilitating the offender to the norms of the society i.e. into law-abiding member. This theory condemns all kinds of corporal punishments4. Though this theory works stupendously for the correction of juveniles and first time criminals, but in the case of a hardened criminal this theory may not work with the effectiveness5. A Prolegomenon “Crime is behaviour or action that is punishable by criminal law. A crime is a public wrong, as opposed to a moral, wrong; it is an offence committed against (and hence punishable by) the state or the community at large. Many crimes are immoral, but not all actions considered immoral are illegal.”6 According to Durkheim, “crime exists in every society which do and do not have laws, courts and the police. -

Report of Activities 2007-08 C
28 Eklavya: Report of Activities 2007-08 C. Publication Programme C.1 Development and Editorial C.1.1 Chakmak Chakmak is a magazine for children (8 to 14 years) and has been published since 1985. Over the years Chakmak has helped to vastly diversify and enrich children’s literature in Hindi, and to induce leading writers and illustrators to write and illustrate for children. Above all, it has had a great success in motivating the children themselves to write, draw and get published. However, we had been feeling for some time that it had fallen into a stereotype and needed to break out of it. So this year Chakmak took a break for about seven months (April to October) and the time was used to reconceptualise and redesign it so as to give it a contemporary look and feel. A large number of people, both experts on children’s literature and ordinary readers, reviewed Chakmak and helped to mould the direction of change. A two-day review workshop was held involving senior litterateurs, science writers, designers etc. It was noted in this workshop that Chakmak had Eklavya: Report of Activities 2007-08 29 always carried material which tried to make children more sensitive towards their environment, enhanced their creativity, and developed an analytic vision in them. The challenge was to also make it appropriate, attractive and meaningful for today’s readers. It was strongly recommended that Chakmak should have a new look, both in terms of content and design. The need for more original writing presented in an attractive fashion, and diversity in content was greatly emphasized. -

TEQIP-II ACTIVITY CALENDAR July-Dec 2013 Volume I, Issue 2
Guru Nanak Dev Engineering College, Ludhiana An Autonomous College under UGC Act - 1956 [2(f) and 12(B)] TEQIP-II ACTIVITY CALENDAR July-Dec 2013 Volume I, Issue 2 Director : Dr. M. S. Saini www.gndec.ac.in TEQIP Coordinator : Dr. J.N. Jha Nearly 15,000 graduate and 3500 Post Graduate Engineers About TEQIP-II have passed out from this college during the last 57 years Introduction: and are at present successfully employed in India & abroad. The College has also been identified as First QIP Centre of Technical Education Quality Improvement Programme Punjab by All India council for Technical Education (TEQIP) was envisaged as a long-term programme of about (AICTE) for Ph.D Programme and is one among the 10-12 years duration to be implemented in 2-3 phases for existing 42 QIP Centres including IITs at National Level. In transformation of the Technical Education System with the last five years, the faculty has published around 225 research World Bank assistance. papers in National/Internal Journals and more than 450 papers in various conferences in India and abroad. Around As per TEQIP design, each phase is required to be designed 25 teachers have attended International conferences and on the basis of lessons learnt from the implementation of an other interactions under DST/AICTE/PTU travel grant earlier phase. TEQIP-I started a reform process in 127 program. More than 40% teachers are having Ph.D degree Institutions. The reform process needs to be sustained and and another 40% teachers are pursuing Ph.D. 35 Research scaled-up for embedding gains in the system and taking the projects, Seminar Grants and STTPs have been granted by transformation to a higher level. -

Liste Des Livres Et Tirés-À-Part Disponibles Au Club
Liste des livres et tirés-à-part disponibles au Club Auteur Titre Date Etagère Cote ACHCAR Gilbert Le Peuple veut: une exploration radicale du soulèvement arabe 2013 Conférences CLUB/STA ADAM Véronique CAIOZZO Anna dir. (chapitre La fabrique du corps humain: la machine modèle du vivant Promotion 2009 CLUB/STA de ZAPPERI Giovanna) AGLIETTA Michel Un New deal pour l'Europe 2013 Conférences CLUB/STA AGLIETTA Michel Sortir de la crise et inventer l'avenir 2014 Conférences CLUB/AGL AHMED SALEM Zekeria Islam politique et changement social en Mauritanie 2013 Promotion 2012-2013 CLUB/2012-13/AHM AHMED SALEM Zekeria Ould Les Trajectoires d'un Etat-frontière. Espaces, évolution politique et transformations sociales en Mauritanie 2011 Promotion 2012-2013 IEA/SAL AKAKPO Yaovi Nunya, N° 2, Décembre 2014. Imagination et Univers de sens. 2014 Instances IEA CLUB/AKA AKAKPO Yaovi Nunya, N° 2, Décembre 2014. Imagination et Univers de sens. 2014 Instances IEA CLUB/AKA AKANDJI-KOMBE Jean-François Egalité et droit social. (Les rencontres sociales de la Sorbonne, Volume 2.) 2014 Promotion 2016-2017 CLUB/2016-17/AKAN AKHILESH AAP-BEETI Biography of Marc Chagall translation in Hindi 2010 Promotion 2011-2012 CLUB/2011-12/AKH AKHILESH ACHAMBHE KA RONA. Essay on art and artists 2010 Promotion 2011-2012 CLUB/2011-12/AKH AKHILESH AKHILIESH: EK SAMVADD (Dialogue on my art with poet Piyush Daiya) 2010 Promotion 2011-2012 CLUB/2011-12/AKH AKHILESH MAQBOOL . A biography of noted indian painter. First edition 2010 Promotion 2011-2012 CLUB/2011-12/AKH AKHILESH MAQBOOL. Second edition 2010 Promotion 2011-2012 CLUB/2011-12/AKH AKHILESH DARASPOTHI. -

Anaemia and Body Mass Index (BMI) of Fisherwomen Inhabiting in Karang Island of Loktak Lake, Manipur (India)
Eurasian Journal of Anthropology Euras J Anthropol 3(2):47−53, 2012 Anaemia and body mass index (BMI) of fisherwomen inhabiting in Karang island of Loktak Lake, Manipur (India) Maishnam Rustam Singh, Karnajit Mangang2 2Department of Anthropology, Manipur University, Manipur, India Received December 3, 2012 Accepted February 5, 2013 Abstract The paper examines the status of anaemia and body mass index (BMI) among fisherwomen of Karang Island Village, Manipur, India. Altogether 180 Meitei fisherwomen of age group 15 to 49 years were chosen for the study. Two anthropometric measurements viz., stature and body weight were taken on each subject. For estimation of haemoglobin level two standard methods namely, haemoglobin colour scale (HCS) and Sahli’s haemoglobinometer were employed. About 70% of the fisherwomen of Karang village are in normal BMI category, while 16 % of them are underweight, 11 % overweight and 3 % obese. The prevalence of anaemia is notably high among the fisherwomen of this village with a frequency of 68.89%. Women with normal BMI and non-anaemic constitute 24.44%. The mean values of haemoglobin concentration measured by HCS and Sahli’s method is 10.82gm/dl and 10.94gm/dl respectively. The two mean values were tested for t-test of significance and found statistically insignificant (t=0.852). The two adopted methods for haemoglobin estimation viz., HCS and Sahli’s method are found reliable. The correlation value of BMI and haemoglobin level shows negative in association (r = 0.060) and the value is found insignificant (t=0.566). The insignificance of this relationship means there is no correlation. -

Sadguru Drishti July-Sept. 2017
Param Pujya Gurudev Shri Ranchhoddasji Maharaj Sadguru Drishti July-Sept. 2017 1 YOUNG ACHIEVER AWARD -- 5th - 6th AUGUST Dr. Gautam Singh Parmar, Head of Department, Cornea, Sadguru Netra Chikitsalaya, Shri Sadguru Seva Sangh Trust, Chitrakoot received Young Achiever Award by Shri. Harshvardhanji, minister for Science and Technology, Government of India, on the occasion of ISCKRS ( Indian society of Cornea and keratorefractive Surgeons) annual conference held at New Delhi on August 5th and 6th, 2017 LIFE TIME ACHIEVEMENT AWARD – 23rd SEPTEMBER It is indeed a proud moment as Dr. B. K. Jain, Trustee & Director of Shri Sadguru Seva Sangh Trust, Chitrakoot was presented with ‘The Life Time Achievement Award’ at the Intraocular Implant & Refractive Society of India (IIRSI) - Annual Conference, New Delhi on September 23, 2017. From the latest innovation to the challenges, the scientific committee of the IIRSI honours people who have contributed immensely in the field of Ophthalmology. Dr. B.K. Jain has made various presentations and papers in the said field. He has also introduced community outreach eye camps, vision centers and operational research in Central India. His vision to put Sadguru Netra Chikitsalaya on the global map of eye care providers truly makes him deserving of the award. Our heartiest congratulations from the entire family of Shri Sadguru Seva Sangh Trust. 2 CHIEF MINISTER’S VISIT -- 10th SEPTEMBER Shri Shivraj Singh Chouhan, Chief Minister of Madhya Pradesh visited Sadguru Netra Chikitsalaya, Shri Sadguru Seva Sangh Trust (SSSST), Chitrakoot on 10th September, 2017. Dr. B.K. Jain, Director and Trustee of SSSST showcased the activities undertaken by the trust to the Chief Minister who appreciated the contributions and assured full co-operation by the State Govt. -

In the Supreme Court of India Civil Appellate Jurisdiction
1 REPORTABLE IN THE SUPREME COURT OF INDIA CIVIL APPELLATE JURISDICTION CIVIL APPEAL NOS. 3681-3682 OF 2020 (Arising out of SLP (C) Nos. 21326-21327 OF 2019) Rattan Singh & Ors. ¼ Appellants Versus Nirmal Gill & Ors. etc. ¼Respondents WITH CIVIL APPEAL NOS. 3683-3684 OF 2020 (Arising out of SLP (C) Nos. 29775-29776 OF 2019) Inder Pal Singh & Anr. ¼ Appellants Versus Nirmal Gill & Ors. etc. ¼Respondents J U D G M E N T A.M. Khanwilkar, J. 1. Leave granted. 2. These appeals take exception to the common Judgment and decree of the High Court of Punjab and Haryana at Chandigarh1, dated 27.05.2019 in R.S.A. Nos. 2901/2012 and 3881/2012, 1 for short, “the High Court” 2 whereby the High Court reversed the concurrent findings of the trial Court and the first appellate Court and decreed the suits of the plaintiff. 3. For convenience, the parties are referred to as per their status in Civil Suit No. 11/2001 before the Court of Civil Judge (Senior Division), Hoshiarpur2. The admitted factual position in the present cases is that one Harbans Singh had married Gurbachan Kaur and fathered Joginder Kaur (plaintiff ± now deceased) in the wedlock. After the demise of Gurbachan Kaur, Harbans Singh married Piar Kaur and in that wedlock, he fathered Gurdial Singh (defendant No. 3), Rattan Singh (defendant No. 4), Narinder Pal Singh (defendant No. 5) and Surjit Singh (defendant No. 6). Harcharan Kaur (defendant No. 1) is the wife of defendant No. 4 and the step sister-in-law of the plaintiff. -

Articles Alert April-June 2013
JUSTICE T.P.S. CHAWLA LIBRARY NATIONAL LAW UNIVERSITY DELHI Articles Alert April-June 2013 National Law Univ., Delhi Articles Check List 30/07/2014 1 Rajan, Amitabh Evolution of information rights jurisprudence. ALL INDIA REPORTER, 100, 2013(April): p 65. Supp. Flag : N Control No. : 12340 2 Gupta, Karn Grievance redressal mechanism under consumer protection act vis-a-vis banking ombudsman scheme, 2002. ALL INDIA REPORTER, 100, 2013(April): p 71. Supp. Flag : N Control No. : 12341 3 Mishra, Vishal Failure to make disclosures required under regulation 3 of sast regulations, 1997 entails penalty despite being... SEBI AND CORPORATE LAWS, 118(3), 2013(April): p 91. Supp. Flag : N Control No. : 12342 4 Chhaba, Ravindra and Chhaba, Shyam Sunder Pledging of shares by promoters: Investor's dilemma. SEBI AND CORPORATE LAWS, 118(3), 2013(April): p 94. Supp. Flag : N Control No. : 12343 5 Singh, Jayantika Alternative investment funds: Venturing out of the fund troubles? SEBI AND CORPORATE LAWS, 118(3), 2013(April): p 101. Supp. Flag : N Control No. : 12344 6 Arora, Sahil and Singh, Chitwan Deep Jurisdictional issues arising under the companies act, 1956: Juxtaposing the conflicting judgments from involving... SEBI AND CORPORATE LAWS, 118(3), 2013(April): p 107. Supp. Flag : N Control No. : 12345 7 Thakur, Rustam Singh and Mitra, Daksh Heralding new generation of investment advisers: SEBI notifies investment advisers regulations, 2013. SEBI AND CORPORATE LAWS, 118(3), 2013(April): p 113. Supp. Flag : N Control No. : 12346 8 Dhawan, Rani Higher education: Quality assuarance and quality enhancement. UNIVERSITY NEWS, 51(13), 2013(April): p 1. -
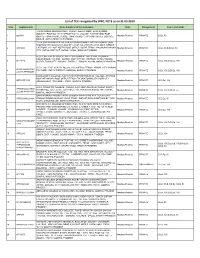
WRC for Website.Xlsx
List of TEIs recognized by WRC, NCTE as on 31.03.2019 S.No. Application ID Name & Address of the Institutions State Management Course and Intake GURU DROAN MAHAVIDYALAY RUN BY JEEVAN AMRIT EDUCATIONAL SOCIETY, PLOT NO- 16/10, STREET NO- 02, VILLAGE- TONK KHURD, POST 1 app3441 OFFICE- TONK KHURD, TEHSIL- TONK KHURD, CITY-TONK KHURD, DISTRICT: Madhya Pradesh PRIVATE B.Ed. 50 DEWAS- 455116, MADHYA PRADESH LATE UMA RAMAN PRATAP BHADUR SINGH MAHAVIDYALAYA RUN BY UMA RAMAN MAHAVIDAYALAYA SOCIETY, PLOT NO: 273/1KH, 281/2, 283/1, STREET: 2 APP2908 CKT ROAD, VILLAGE: KOTHI, POST OFFICE: KOTHI, TEHSIL: RAGHURAJ NAGAR, Madhya Pradesh PRIVATE B.Ed. 50,D.El.Ed. 50 CITY: SATNA, DISTRICT: SATNA- 485666, MADHYA PRADESH A S E COLLEGE OF EDUCATION PLOT NUMBER : 648, STREET NUMBER : NANDNIROAD, VILLAGE : NANDNI, POST OFFICE : RATIBAD, TEHSIL/TALUKA : 3 APP1876 HUZUR, TOWN/CITY : BHOPAL, DISTRICT : BHOPAL 462044, MADHYA PRADESH Madhya Pradesh PRIVATE B.Ed. 100,D.El.Ed. 100 A.D.S. COLLEGE SURVEY NO.610, VILLAGE/P.O./TEHSIL- AMBAH, CITY- AMBAH, APW07644/223767/ 4 PIN CODE- 476111, DISTRICT- MORENA, MADHYA PRADESH Madhya Pradesh PRIVATE B.Ed. 100,D.El.Ed. 100 222338/APW06290 EXCELLENCY COLLEGE PLOT/KHASRA/STREET NO.519/2, VILLAGE – BY PASS ROAD KE ANDAR, POST OFFICE/TEHSIL/TALUKA/TOWN/CITY/DISTRICT – 5 WRCAPP3358 ASHOKNAGAR, PIN CODE – 473331, MADHYA PRADESH Madhya Pradesh PRIVATE D.El.Ed. 100 A.D.S. GROUP OF COLLEGE (RUN BY AJAR AMAR SHIKSHA PRASAR SAMITI), APW00822/223810/ 6 KHASRA NO. 343/2, 343/3, 345/1/2/KA/2, VILL-PARWALIA SADAK, TEH-HUZUR,, Madhya Pradesh PRIVATE B.Ed. -

English Language Proficiency: the Only Essential Component for Successful Startups?
International Journal of Applied Business and Economic Research Volume 17, Number 3, 2019, ISSN : 0972-7302 available at http: www.serialsjournals.com ENGLISH LANGUAGE PROFICIENCY: THE ONLY ESSENTIAL COMPONENT FOR SUCCESSFUL StARTUPS? Ramnita Saini Sharda Associate Professor , Department of English, Hans Raj Mahila Maha Vidyalaya, Jalandhar Abstract: Start-up is the buzz word of the present day’s business corridors. But the success stories are very few. For this slow and negligible success rate, who do we blame? Is it the Government, educational institutions, supporting agencies, lack of innovation or a failed communication that is responsible for the failure? Since the number of failures is larger than successful ventures, it is important to understand the possible reasons for failure than to find markers for sure shot success. Besides other reasons for the negligible growth rate of startups, failed communication is one major cause. The paper delves into the role of effective communication right from seed stage to steady stage. For effective communication is it important to have English Language Proficiency? Some strategists are of the view that one can’t budge an iota without the proficiency in the English language. On the other hand, certain scholars vouch for the effectiveness of regional languages in the digital age. As per FICCI KMPG report 88 percent Indi- ans are non English Speaking! Does this mean that we can ignore English language completely? With these multifarious voices creating an unbearable cacophony, we need to understand the dynamics of the Indian market and the communicative language strategies that can facilitate the nouveau entrepreneur’s startups and make the venture successful. -
And Truth-Event: Reflec- Tions on Muktibodhian Creative-Process
Language, Literature, and Interdisciplinary Studies (LLIDS) ISSN: 2547-0044 http://ellids.com/archives/2019/03/2.3-Bali.pdf CC Attribution-No Derivatives 4.0 International License http://ellids.com/ The ‘Three Moments of Art’ and Truth-Event: Reflec- tions on Muktibodhian Creative-Process Anup Kumar Bali Introduction Poetry has its own politics within the ambit of its own specific- ity. But to conceive any phenomena only in an identitarian form1 is precisely an indication towards the fundamental problem of envision- ing phenomena and processes in dichotomized distinct forms that function according to the logic of binary oppositions. This problematic is pertinent to envision the relationship between poetry and politics not as dichotomized distinct processes rather as mutually constitutive ones. On the one hand, dominant ideological manoeuvrings reduce po- etry as an instrument for ‘political’ struggles in ‘political’ movements whereas we have also been the spectator of art for art’s sake kind of conception which clenches poetry within the identitarian fortifications of aestheticism on the other extreme. While shattering these kinds of delusional dichotomies, this paper intends to illumine art of poetry as 1Here identitarian stands for identification of phenomenon without reflecting upon the processuality that leads to phenomenal appearance. German philosopher, Theo- dor W. Adorno, reflects upon this as identity thinking which privileges positivity, origin, fixity, and most importantly immanence. With his immanent critique of iden- titarian thinking—which is the consequent result of idealist dialectic of Hegel—he offers Negative dialectics. In light of this discussion, poetry and politics are not two distinct phenomena as binary oppositions functional only at certain junctures rather the entwinement of poetry and politics as processual unfolding envisages at diverse junctures. -

« Ce Texte Est Disponible Sous Les Termes De La Licence Creative Commons Paternité-Partage Des Conditions Initiales À L'identique 3.0 Unported (CC-BY-SA) »
« Ce texte est disponible sous les termes de la Licence Creative commons Paternité-Partage des Conditions Initiales à l'Identique 3.0 Unported (CC-BY-SA) » Come over O Bada Dev, sit in the dense shade of the Saja tree. Come, create the world, create it once again. Where the village comes to an end keep vigil O Mahrilin Devi. Let no illness, no disease head our way, stop it right there. At the edge of the forest stand guard O Ghurri Dev. Keep us safe, keep us safe from the wild beast. At the village crossroads stay put O Tipthain Dev. Confer at all given hours on us a boon of plenty. Sprawl yourself O Dehri Dev over our humble doorway. Let no female demon step into the house. Come, make your home O Chulha Dev in the freshly smeared chulha. Fill with the most delicious taste every speck of food. When night falls in desolate streets make your round O Ratmai Murkhuri. So the weary humans can sleep, so they can sleep in peace. 1. It wasn’t anything new for the residents of Patangarh to leave their village and live and work elsewhere. The father of Prasad Singh Shyam left for Shillong with Verrier Elvin about 40-50 years ago. Elvin went there as an advisor to the government. Prasad’s father was to look after him. He lived there and every month sent a small amount of money to his family. This amount was barely enough to sustain the family which was extremely poor. ‘They were so poor, Saheb, that it’s difficult to describe their condition.’ The family had six brothers and four sisters and no land to speak of.