A Rapid Assessment of Natural Environments in the Maldives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bibliography Database of Living/Fossil Sharks, Rays and Chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the Year 2016
www.shark-references.com Version 13.01.2017 Bibliography database of living/fossil sharks, rays and chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the year 2016 published by Jürgen Pollerspöck, Benediktinerring 34, 94569 Stephansposching, Germany and Nicolas Straube, Munich, Germany ISSN: 2195-6499 copyright by the authors 1 please inform us about missing papers: [email protected] www.shark-references.com Version 13.01.2017 Abstract: This paper contains a collection of 803 citations (no conference abstracts) on topics related to extant and extinct Chondrichthyes (sharks, rays, and chimaeras) as well as a list of Chondrichthyan species and hosted parasites newly described in 2016. The list is the result of regular queries in numerous journals, books and online publications. It provides a complete list of publication citations as well as a database report containing rearranged subsets of the list sorted by the keyword statistics, extant and extinct genera and species descriptions from the years 2000 to 2016, list of descriptions of extinct and extant species from 2016, parasitology, reproduction, distribution, diet, conservation, and taxonomy. The paper is intended to be consulted for information. In addition, we provide information on the geographic and depth distribution of newly described species, i.e. the type specimens from the year 1990- 2016 in a hot spot analysis. Please note that the content of this paper has been compiled to the best of our abilities based on current knowledge and practice, however, -

(Cestoda) from the Mangrove Whipray, Urogymnus Granulatus (Myliobatiformes: Dasyatidae), from the Solomon Islands and Northern Australia
Institute of Parasitology, Biology Centre CAS Folia Parasitologica 2017, 64: 004 doi: 10.14411/fp.2017.004 http://folia.paru.cas.cz Research Article A new genus with two new species of lecanicephalidean tapeworms (Cestoda) from the mangrove whipray, Urogymnus granulatus (Myliobatiformes: Dasyatidae), from the Solomon Islands and northern Australia Kaylee S. Herzog and Kirsten Jensen Department of Ecology and Evolutionary Biology, and the Biodiversity Institute, University of Kansas, Lawrence, Kansas, USA Abstract: A new lecanicephalidean genus is erected for cestodes previously recognised as “New Genus 12” (Polypocephalidae) in a phylogenetic analysis of the interrelationship of members of this order. Examination of the cestode fauna of the mangrove whipray, Urogymnus granulatus (Macleay) (Myliobatiformes: Dasyatidae) from the Solomon Islands and northern Australia revealed the existence of specimens representing two new species, consistent in morphology with “New Genus 12.” Corollapex gen. n. is unique among the 24 valid lecanicephalidean genera in its possession of an apical organ in the form of an external retractable central disk surrounded by eight concave muscular, membrane-bound pads and an internal heterogeneous glandular component. The two new spe- cies described herein, Corollapex cairae sp. n. (type species) and Corollapex tingoi sp. n., differ from one another in overall size and number of mature and immature proglottids, and are noted to demonstrate a differential distribution between mature and juvenile host individuals. Additional species diversity in the new genus, beyond C. cairae sp. n., C. tingoi sp. n., and “New Genus 12 n. sp. 1” of Jensen et al. (2016) is suggested. Corollapex gen. n. appears to be restricted to dasyatid hosts in the Indo-West Pacific region. -

Checklist of Philippine Chondrichthyes
CSIRO MARINE LABORATORIES Report 243 CHECKLIST OF PHILIPPINE CHONDRICHTHYES Compagno, L.J.V., Last, P.R., Stevens, J.D., and Alava, M.N.R. May 2005 CSIRO MARINE LABORATORIES Report 243 CHECKLIST OF PHILIPPINE CHONDRICHTHYES Compagno, L.J.V., Last, P.R., Stevens, J.D., and Alava, M.N.R. May 2005 Checklist of Philippine chondrichthyes. Bibliography. ISBN 1 876996 95 1. 1. Chondrichthyes - Philippines. 2. Sharks - Philippines. 3. Stingrays - Philippines. I. Compagno, Leonard Joseph Victor. II. CSIRO. Marine Laboratories. (Series : Report (CSIRO. Marine Laboratories) ; 243). 597.309599 1 CHECKLIST OF PHILIPPINE CHONDRICHTHYES Compagno, L.J.V.1, Last, P.R.2, Stevens, J.D.2, and Alava, M.N.R.3 1 Shark Research Center, South African Museum, Iziko–Museums of Cape Town, PO Box 61, Cape Town, 8000, South Africa 2 CSIRO Marine Research, GPO Box 1538, Hobart, Tasmania, 7001, Australia 3 Species Conservation Program, WWF-Phils., Teachers Village, Central Diliman, Quezon City 1101, Philippines (former address) ABSTRACT Since the first publication on Philippines fishes in 1706, naturalists and ichthyologists have attempted to define and describe the diversity of this rich and biogeographically important fauna. The emphasis has been on fishes generally but these studies have also contributed greatly to our knowledge of chondrichthyans in the region, as well as across the broader Indo–West Pacific. An annotated checklist of cartilaginous fishes of the Philippines is compiled based on historical information and new data. A Taiwanese deepwater trawl survey off Luzon in 1995 produced specimens of 15 species including 12 new records for the Philippines and a few species new to science. -

Malaysia National Plan of Action for the Conservation and Management of Shark (Plan2)
MALAYSIA NATIONAL PLAN OF ACTION FOR THE CONSERVATION AND MANAGEMENT OF SHARK (PLAN2) DEPARTMENT OF FISHERIES MINISTRY OF AGRICULTURE AND AGRO-BASED INDUSTRY MALAYSIA 2014 First Printing, 2014 Copyright Department of Fisheries Malaysia, 2014 All Rights Reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic, mechanical, including photocopy, recording, or any information storage and retrieval system, without prior permission in writing from the Department of Fisheries Malaysia. Published in Malaysia by Department of Fisheries Malaysia Ministry of Agriculture and Agro-based Industry Malaysia, Level 1-6, Wisma Tani Lot 4G2, Precinct 4, 62628 Putrajaya Malaysia Telephone No. : 603 88704000 Fax No. : 603 88891233 E-mail : [email protected] Website : http://dof.gov.my Perpustakaan Negara Malaysia Cataloguing-in-Publication Data ISBN 978-983-9819-99-1 This publication should be cited as follows: Department of Fisheries Malaysia, 2014. Malaysia National Plan of Action for the Conservation and Management of Shark (Plan 2), Ministry of Agriculture and Agro- based Industry Malaysia, Putrajaya, Malaysia. 50pp SUMMARY Malaysia has been very supportive of the International Plan of Action for Sharks (IPOA-SHARKS) developed by FAO that is to be implemented voluntarily by countries concerned. This led to the development of Malaysia’s own National Plan of Action for the Conservation and Management of Shark or NPOA-Shark (Plan 1) in 2006. The successful development of Malaysia’s second National Plan of Action for the Conservation and Management of Shark (Plan 2) is a manifestation of her renewed commitment to the continuous improvement of shark conservation and management measures in Malaysia. -

ENVIRONMENTAL IMPACT ASSESSMENT for the Proposed Resort Development in Kabaalifaru Resort, Shaviyani Atoll, Maldives
ENVIRONMENTAL IMPACT ASSESSMENT For the Proposed resort development in Kabaalifaru Resort, Shaviyani Atoll, Maldives Proposed by Ali Shareef Signature: Prepared by Aslam Shakir For Water Solutions Pvt. Ltd., Maldives September 2007 EIA for Kanbaalifaru, Vol 1, Rev 0 Page | ii Table of Contents 1 INTRODUCTION .......................................................................................................................... 1 1.1 AIMS AND OBJECTIVES OF THE EIA .................................................................................................. 1 1.2 TERMS OF REFERENCE AND SCOPE FOR THIS EIA ............................................................................ 1 1.3 METHODOLOGIES .............................................................................................................................. 2 1.4 EXECUTION OF THE EIA .................................................................................................................... 3 2 PROJECT DESCRIPTION .......................................................................................................... 4 2.1 PROJECT PROPONENT ........................................................................................................................ 4 2.2 PROJECT LOCATION ........................................................................................................................... 4 2.3 PROJECT JUSTIFICATION .................................................................................................................... 5 2.4 PROJECT -

E-Brochure-Resort-SLMD.Pdf
YOUR SANCTUARY BEYOND PARADISE Experience an unforgettable journey. Arrive in style to the iconic Shangri-La’s Villingili Resort & Spa, a secluded retreat, the only resort south of the equator. From the moment you land, discover a world of sophistication, a pinnacle of luxury promising the experience of a lifetime that is beyond compare. ARRIVING TO BLISS MALDIVES Addu Atoll AIRSIDE Shangri-La’s Villingili Resort & Spa, Maldives TO POOLSIDE IN Heron Island 5 MINUTES Malé International Airport Transfer from your international flight at Malé Airport where you will be personally welcomed by our resort representative. 5 minutes by speedboat Refresh, relax and unwind in the comfort of Mooninaa Lounge whilst we coordinate your connecting Maldivian Airlines flight. Touch down at the fully serviced terminal of Gan International Airport, ideally Gan International located just minutes from the resort. Airport Alternatively, you can choose to fly with SriLankan Airlines and take a direct flight from Colombo to Gan Airport for a seamless arrival to this spectacular island. For the ultimate experience book your own private jet, arrive in style and on your own preferred schedule. A complimentary speedboat ride connects the Gan Airport to Villingili in just five minutes, providing a quick and efficient transition to this exclusive resort. Equator Addu Atoll A UNIQUE ISLAND RETREAT NATURAL HAVEN Villingili Island, a stunning jewel in the heart of the Indian Ocean. A natural beach-soaked paradise rising out of the azure waters, and the largest dedicated resort island in the Maldives. Positioned at the southernmost point of the archipelago, the beautiful heart shaped Addu Atoll is protected by a stunning, tropical coral reef. -

Land Acquisition and Resettlement Due Diligence Report ______
Land Acquisition and Resettlement Due Diligence Report ____________________________________________________________________________ Document Stage: Draft Project Number: 51077 March 2018 Republic of the Maldives: Greater Malé Environmental Improvement and Waste Management Project - Thulusdhoo Island Waste Management Improvements Subproject This Due Diligence Report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. CURRENCY EQUIVALENTS (as of 15 March 2018) Currency unit = Rufiyaa (Rf) Rf1.00 = $0.065 USD USD 1.00 = Rf15.449 ABBREVIATIONS ADB - Asian Development Bank DDR - due diligence report IWMC - Island Waste Management Centre SWM - Solid waste management tpd - tons per day WAMCO - Waste Management Corporation Limited CONTENTS I. INTRODUCTION .................................................................................................................1 A. Project Background ..........................................................................................................1 B. Project Description ...........................................................................................................1 -

Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997
The IUCN Species Survival Commission Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 Edited by Sarah L. Fowler, Tim M. Reed and Frances A. Dipper Occasional Paper of the IUCN Species Survival Commission No. 25 IUCN The World Conservation Union Donors to the SSC Conservation Communications Programme and Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 The IUCN/Species Survival Commission is committed to communicate important species conservation information to natural resource managers, decision-makers and others whose actions affect the conservation of biodiversity. The SSC's Action Plans, Occasional Papers, newsletter Species and other publications are supported by a wide variety of generous donors including: The Sultanate of Oman established the Peter Scott IUCN/SSC Action Plan Fund in 1990. The Fund supports Action Plan development and implementation. To date, more than 80 grants have been made from the Fund to SSC Specialist Groups. The SSC is grateful to the Sultanate of Oman for its confidence in and support for species conservation worldwide. The Council of Agriculture (COA), Taiwan has awarded major grants to the SSC's Wildlife Trade Programme and Conservation Communications Programme. This support has enabled SSC to continue its valuable technical advisory service to the Parties to CITES as well as to the larger global conservation community. Among other responsibilities, the COA is in charge of matters concerning the designation and management of nature reserves, conservation of wildlife and their habitats, conservation of natural landscapes, coordination of law enforcement efforts as well as promotion of conservation education, research and international cooperation. -

ENVIRONMENTAL IMPACT ASSESSMENT for the Construction of a Slipway in Goidhoo Island, Shaviyani Atoll, Maldives
ENVIRONMENTAL IMPACT ASSESSMENT For the construction of a slipway in Goidhoo Island, Shaviyani Atoll, Maldives Photo: Water Solutions Proposed by: 3L Construction PVT.LTD Prepared by: Hassan Shah (EIA P02/2007) For Water Solutions Pvt. Ltd., Maldives May 2018 BLANK PAGE EIA For the construction of a slipway in Goidhoo Island, Shaviyani Atoll 1 Table of contents 1 Table of contents ...................................................................................................... 3 2 List of Figures and Tables ........................................................................................ 7 3 Declaration of the consultants .................................................................................. 8 4 Proponents Commitment and Declaration ............................................................... 9 5 Non-Technical Summary ....................................................................................... 13 6 Introduction ............................................................................................................ 15 6.1 Structure of the EIA ........................................................................................... 15 6.2 Aims and Objectives of the EIA ........................................................................ 15 6.3 EIA Implementation .......................................................................................... 15 6.4 Rational for the formulation of alternatives ....................................................... 15 6.5 Terms of Reference........................................................................................... -

2020/016 Dated 28 February 2020 in the Language and Format in Which They Were Received
AC31 Doc. 25 Annex 2 Compilation of responses from Parties to Notification to the Parties No. 2020/016 dated 28 February 2020 in the language and format in which they were received Contents Cambodia ………………………………………………………………………………………………………………………………………....…1 Canada ………………………………………………………………………………………………………………………………..………..……..3 Colombia …………………………………………………………………………………………………………………………………….………..5 Costa Rica …………………………………………………………………………………………………………………………..………..………15 Croatia ……………………………………………………………………………………………………………………………..………..……….35 European Union ………………………………………………………………………………………………………..……………..………….36 Indonesia ………………………………………………………………………………………………………………………………………..…..43 Israel ……………………………………………………………………………………………………………………………………………..…….51 Italy ……………………………………………………………………………………………………………………………………………………..55 Japan ……………………………………………………………………………………………………………………………………………….….60 Mexico ………………………………………………………………………………………………………………………………………………..66 Monaco ……………………………………………………………………………………………………………………………………………….69 Netherlands …………………………………………………………………………………………………………………………………………73 New Zealand ………………………………………………………………………………………………………………………………………..77 Oceania Parties …………………………………………………………………………………………………………………………………….79 Peru ……………………………………………………………………………………………………………………………………………………..83 Senegal ………………………………………………………………………………………………………………………………………………..89 Thailand ……………………………………………………………………………………………………………………………………………….91 United States of America ……………………………………………………………………………………………………………………146 Cambodia AC31 Doc. 25 Annex 2 Canada Canadian Response to CITES Notification 2020/016 -

37327 Public Disclosure Authorized
37327 Public Disclosure Authorized REPUBLIC OF THE MALDIVES Public Disclosure Authorized TSUNAMI IMPACT AND RECOVERY Public Disclosure Authorized Public Disclosure Authorized JOINT NEEDS ASSESSMENT WORLD BANK - ASIAN DEVELOPMENT BANK - UN SYSTEM ki QU0 --- i 1 I I i i i i I I I I I i Maldives Tsunami: Impact and Recovery. Joint Needs Assessment by World Bank-ADB-UN System Page 2 ABBREVIATIONS ADB Asian Development Bank DRMS Disaster Risk Management Strategy GDP Gross Domestic Product GoM The Government of Maldives IDP Internally displaced people IFC The International Finance Corporation IFRC International Federation of Red Cross IMF The International Monetary Fund JBIC Japan Bank for International Cooperation MEC Ministry of Environment and Construction MFAMR Ministry of Fisheries, Agriculture, and Marine Resources MOH Ministry of Health NDMC National Disaster Management Center NGO Non-Governmental Organization PCB Polychlorinated biphenyls Rf. Maldivian Rufiyaa SME Small and Medium Enterprises STELCO State Electricity Company Limited TRRF Tsunami Relief and Reconstruction Fund UN United Nations UNFPA The United Nations Population Fund UNICEF The United Nations Children's Fund WFP World Food Program ACKNOWLEDGEMENTS This report was prepared by a Joint Assessment Team from the Asian Development Bank (ADB), the United Nations, and the World Bank. The report would not have been possible without the extensive contributions made by the Government and people of the Maldives. Many of the Government counterparts have been working round the clock since the tsunami struck and yet they were able and willing to provide their time to the Assessment team while also carrying out their regular work. It is difficult to name each and every person who contributed. -
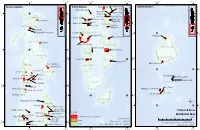
Protected Areas Distribution
73°0'0"E 74°0'0"E 73°0'0"E 74°0'0"E 73°0'0"E 74°0'0"E Northern Maldives Central Maldives Rasfari beyru Huraa Mangrove Area Southern Maldives Laamu Atoll Rasdhoo Madivaru Girifushi Thila Banana Reef Nassimo Thila 7°0'0"N 7°0'0"N Kuda Haa Lions Head Hans Hass Place; HP Reef Haa Alifu Atoll Mayaa Thila &% Kari beyru Thila Baarah Kulhi Emboodhoo Alifu Alifu Atoll Kanduolhi Orimas Thila 4°0'0"N Kaafu Atoll 4°0'0"N Haa Dhaalu Atoll Fish Head Guraidhoo &% Kanduolhi &% Keylakunu Neykurendhoo Mangrove Hurasdhoo Alifu Dhaalu Atoll 1°0'0"N 1°0'0"N Kudarah Thila Hithaadhoo Rangali Kandu Dhevana Kandu Shaviyani Atoll &% Farukolhu South Ari Atoll MPA Vaavu Atoll Filitheyo Kandu Gaafu Alifu Atoll Vattaru Kandu 6°0'0"N 6°0'0"N Faafu Atoll Noonu Atoll Gaafu Dhaalu Atoll Fushee Kandu Meemu Atoll 3°0'0"N Hakuraa Thila 3°0'0"N Kuredu Express Dhigulaabadhoo Raa Atoll &% Dhaalu Atoll &% Fushivaru Thila 0°0'0" 0°0'0" &% Bathala Region Anemone City &% Lhaviyani Atoll Mendhoo Region Angafaru Thoondi Area Dhandimagu Kilhi &% Maahuruvalhi &% &% &% &% Hanifaru Bandaara Kilhi Thaa Atoll Gnaviyani Atoll Baa Atoll Dhigali Haa &% 5°0'0"N Olhugiri 5°0'0"N Kan'di hera The Wreck of Corbin&% &% Hithadhoo Protected Area Goidhoo Koaru &% Seenu Atoll Mathifaru Huraa British Loyalty 2°0'0"N 2°0'0"N Laamu Atoll Makunudhoo channel &% Kaafu Atoll ¶ Rasfari beyru&% Huraa Mangrove Area 1°0'0"S 1°0'0"S &% Rasdhoo Madivaru &% Girifushi Thila &% Protected Areas &% Nassimo Thila &% Legend Kuda Haa &%Male' CityBanana Reef Kari beyru Thila &% &% Distribution Map Mayaa Thila Lions Head Hans Hass Place Protected Areas 2019 (Total 50 sites) 0 25 50 100 Km &% &% &% Sources: EPA 2019 Alifu Alifu Atoll Emboodhoo Islands Kanduolhi Map version Date: 30/06/2019 &% Orimas Thila Projection: Transverse Mercator (UTM Zone 43 N); 4°0'0"N &% 4°0'0"N Reefs Prepared by: Ministry of Environment, Maldives Fish Head &%Guraidhoo Kanduolhi Horizontal Datum: WGS84; 73°0'0"E 74°0'0"E 73°0'0"E 74°0'0"E 73°0'0"E 74°0'0"E.