Forks of the Credit Provincial Park
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
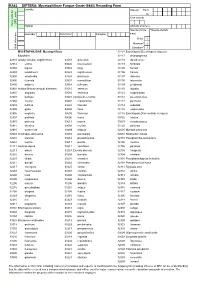
Ra82 Diptera
RA82 DIPTERA: Mycetophilinae Fungus Gnats (6480) Recording Form Locality Date(s) from: to: Vice county GPS users Grey cells for Habitat Altitude (metres) Source (circle *Source details Recorder Determiner Compiler one) Field 1 Museum* 2 Grid reference Literature* 3 MYCETOPHILIDAE: Mycetophilinae 33101 Exechiopsis (Exechiopsis) clypeata Exechiini 33117 dryaspagensis 32801 Allodia (Allodia) anglofennica 33519 griseolum33118 dumitrescae 32912 embla 33526 intermedium33119 fimbriata 32802 lugens 33522 kingi33120 furcata 32803 lundstroemi 33523 nigrofuscum33106 hammi 32804 ornaticollis 33524 proximum33107 indecisa 32806 truncata 33527 rosmellitum33108 intersecta 32805 zaitzevi 33514 ruficorne33109 jenkinsoni 32901 Allodia (Brachycampta) alternans 33515 serenum33110 ligulata 32910 angulata 33516 sericoma33121 magnicauda 32903 barbata 33601 Cordyla brevicornis33112 pseudindecisa 32904 czernyi 33602 crassicornis33113 pulchella 32909 foliifera 33603 fasciata33114 subulata 32905 grata 33604 fissa33115 unguiculata 32906 neglecta 33605 flaviceps33116 Exechiopsis (Xenexechia) crucigera 32907 pistillata 33606 fusca33002 leptura 32915 protenta 33613 insons33003 membranacea 32914 silvatica 33608 murina33122 pollicata 32916 westerholti 33609 nitidula32605 Myrosia maculosa 32602 Allodiopsis domestica 33610 parvipalpis32601 Notolopha cristata 32610 korolevi 33614 pseudomurina32701 Pseudexechia aurivernica 32607 rustica 33611 pusilla32706 monica 32212 Anatella alpina 33612 semiflava32705 parallela 32213 ankeli 33201 Exechia bicincta32703 trisignata 32216 bremia -

Impact of Imidacloprid and Horticultural Oil on Nonâ•Fitarget
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 8-2007 Impact of Imidacloprid and Horticultural Oil on Non–target Phytophagous and Transient Canopy Insects Associated with Eastern Hemlock, Tsuga canadensis (L.) Carrieré, in the Southern Appalachians Carla Irene Dilling University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Entomology Commons Recommended Citation Dilling, Carla Irene, "Impact of Imidacloprid and Horticultural Oil on Non–target Phytophagous and Transient Canopy Insects Associated with Eastern Hemlock, Tsuga canadensis (L.) Carrieré, in the Southern Appalachians. " Master's Thesis, University of Tennessee, 2007. https://trace.tennessee.edu/utk_gradthes/120 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Carla Irene Dilling entitled "Impact of Imidacloprid and Horticultural Oil on Non–target Phytophagous and Transient Canopy Insects Associated with Eastern Hemlock, Tsuga canadensis (L.) Carrieré, in the Southern Appalachians." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Entomology and Plant Pathology. Paris L. Lambdin, Major Professor We have read this thesis and recommend its acceptance: Jerome Grant, Nathan Sanders, James Rhea, Nicole Labbé Accepted for the Council: Carolyn R. -

Tachinid (Diptera: Tachinidae) Parasitoid Diversity and Temporal Abundance at a Single Site in the Northeastern United States Author(S): Diego J
Tachinid (Diptera: Tachinidae) Parasitoid Diversity and Temporal Abundance at a Single Site in the Northeastern United States Author(s): Diego J. Inclan and John O. Stireman, III Source: Annals of the Entomological Society of America, 104(2):287-296. Published By: Entomological Society of America https://doi.org/10.1603/AN10047 URL: http://www.bioone.org/doi/full/10.1603/AN10047 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. CONSERVATION BIOLOGY AND BIODIVERSITY Tachinid (Diptera: Tachinidae) Parasitoid Diversity and Temporal Abundance at a Single Site in the Northeastern United States 1 DIEGO J. INCLAN AND JOHN O. STIREMAN, III Department of Biological Sciences, 3640 Colonel Glenn Highway, 235A, BH, Wright State University, Dayton, OH 45435 Ann. Entomol. Soc. Am. 104(2): 287Ð296 (2011); DOI: 10.1603/AN10047 ABSTRACT Although tachinids are one of the most diverse families of Diptera and represent the largest group of nonhymenopteran parasitoids, their local diversity and distribution patterns of most species in the family are poorly known. -

To Us Insectometers, It Is Clear That Insect Decline in Our Costa Rican
SPECIAL FEATURE: PERSPECTIVE To us insectometers, it is clear that insect decline in our Costa Rican tropics is real, so let’sbekindto SPECIAL FEATURE: PERSPECTIVE the survivors Daniel H. Janzena,1 and Winnie Hallwachsa Edited by David L. Wagner, University of Connecticut, and accepted by Editorial Board Member May R. Berenbaum November 11, 2020 (received for review April 6, 2020) We have been field observers of tropical insects on four continents and, since 1978, intense observers of caterpillars, their parasites, and their associates in the 1,260 km2 of dry, cloud, and rain forests of Area´ de Conservaci ´onGuanacaste (ACG) in northwestern Costa Rica. ACG’s natural ecosystem restoration began with its national park designation in 1971. As human biomonitors, or “insectometers,” we see that ACG’s insect species richness and density have gradually declined since the late 1970s, and more intensely since about 2005. The overarching perturbation is climate change. It has caused increasing ambient tempera- tures for all ecosystems; more erratic seasonal cues; reduced, erratic, and asynchronous rainfall; heated air masses sliding up the volcanoes and burning off the cloud forest; and dwindling biodiversity in all ACG terrestrial ecosystems. What then is the next step as climate change descends on ACG’s many small-scale successes in sustainable biodevelopment? Be kind to the survivors by stimulating and facilitating their owner societies to value them as legitimate members of a green sustainable nation. Encourage national bioliteracy, BioAlfa. climate change | BioAlfa | conservation by rewilding | biodevelopment | insect decline As “insectometers,” also known as human biomoni- eventually focused into Area´ de Conservaci ´onGuana- tors, we have watched the conspicuous ongoing de- caste (ACG) in northwestern Costa Rica (8–13) (Fig. -

Two New, Brachypterous Limnellia Species from the Venezuelan Andes (Diptera: Ephydridae)
Zootaxa 4144 (3): 301–315 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2016 Magnolia Press ISSN 1175-5334 (online edition) http://doi.org/10.11646/zootaxa.4144.3.1 http://zoobank.org/urn:lsid:zoobank.org:pub:B73CFE90-BDF1-47EA-BBD6-52A8DB2B144C Two new, brachypterous Limnellia species from the Venezuelan Andes (Diptera: Ephydridae) DANIEL N. R. COSTA1, MARCOANDRE SAVARIS2, LUCIANE MARINONI2 & WAYNE N. MATHIS 3 1Fellowship of Departamento de Zoologia, Universidade Federal do Paraná, Jardim das Américas, 81531-980 - Curitiba, Paraná, Brazil. E-mail: [email protected] 2Departamento de Zoologia, Universidade Federal do Paraná, Jardim das Américas, 81531-980 - Curitiba, Paraná, Brazil. E-mails: [email protected] and [email protected] 3Department of Entomology, Smithsonian Institution, NHB 169, PO Box 37012, Washington, D.C. 20013-7012, USA. E-mail: [email protected] Abstract Two new, brachypterous species of Limnellia are described from specimens collected in the Venezuelan Andes: L. vounitis (Trujillo: Bocon, La Cristalina (Andes; 09°14.7′N, 70°19.1′W; 2500 m)) and L. flavifrontis (Mérida: Mérida, Sierra Ne- vada National Park (Laguna Negra; 8°47.1'N; 70°48.4'W; 3300 m)). To facilitate identification of these unusual species, we have included a diagnosis of the tribe Scatellini and of the genus Limnellia and have also provided an annotated key to the South American genera of this tribe. The descriptions are supplemented with illustrations, photographs, and scan- ning electron micrographs of external structures and structures of the male terminalia. Key words: Shore flies, Ephydrinae, Scatellini, L. flavifrontis, L. -

1 an Example of Parasitoid Foraging: Torymus Capite
1 AN EXAMPLE OF PARASITOID FORAGING: TORYMUS CAPITE (HUBER; HYMEMOPTERA: TORYMIDAE [CHALCIDOIDEA]) ATTACKING THE GOLDENROD GALL-MIDGE ASTEROMYIA CARBONIFERA (O. S.; DIPTERA: CECIDOMYIIDAE) Richard F. Green Department of Mathematics and Statistics University of Minnesota Duluth Duluth, MN 55812 U. S. A. INTRODUCTION Van Alphen and Vet (1986) refer to the work of Arthur E. Weis (1983) on a torymid wasp that attacks a gall midge on goldenrod. This system seems to be quite well-studied, particularly, but not exclusively, by Weis. Van Alphen and Vet point out that the parasitoids tend to attack about the same proportion of hosts in patches (galls) with varying numbers of hosts. This has implications for the foraging strategy that the parasitoids use. In this note I want to do three things: (1) outline the basic biology of the organisms involved, (2) describe the results of a foraging experiment conducted by Weis (1983), and (3) interpret the results in terms of Oaten’s stochastic model of optimal foraging. Arthur E. Weis is coauthor of a book (Abrahamson and Weis 1997) on the biology of a three trophic-level system involving a goldenrod stem-gall maker Eurosta solidaginis, its host plant and its enemies. The work described here is earlier work, done an a different species. The biology of the system The species of greatest interest are the torymid parasitoid Torymus capite, which is a larval parasitoid of the gall midge Asteromyia carbonifera, which itself makes blister-like galls on the leaves of goldenrod, especially Canadian goldenrod, Solidgo canadiensis L. (Compositae). There are three generations of gall midge (and its parasitoids) each year. -

Somerset's Ecological Network
Somerset’s Ecological Network Mapping the components of the ecological network in Somerset 2015 Report This report was produced by Michele Bowe, Eleanor Higginson, Jake Chant and Michelle Osbourn of Somerset Wildlife Trust, and Larry Burrows of Somerset County Council, with the support of Dr Kevin Watts of Forest Research. The BEETLE least-cost network model used to produce Somerset’s Ecological Network was developed by Forest Research (Watts et al, 2010). GIS data and mapping was produced with the support of Somerset Environmental Records Centre and First Ecology Somerset Wildlife Trust 34 Wellington Road Taunton TA1 5AW 01823 652 400 Email: [email protected] somersetwildlife.org Front Cover: Broadleaved woodland ecological network in East Mendip Contents 1. Introduction .................................................................................................................... 1 2. Policy and Legislative Background to Ecological Networks ............................................ 3 Introduction ............................................................................................................... 3 Government White Paper on the Natural Environment .............................................. 3 National Planning Policy Framework ......................................................................... 3 The Habitats and Birds Directives ............................................................................. 4 The Conservation of Habitats and Species Regulations 2010 .................................. -

018 PN 18.Pdf (No
Title Psocid News : The Psocidologists' Newsletter Author(s) Yoshizawa, Kazunori Doc URL http://hdl.handle.net/2115/35519 Type other Note edited by Kazunori Yoshizawa at the Systematic Entomology, Faculty of Agriculture, Hokkaido University Additional Information There are other files related to this item in HUSCAP. Check the above URL. File Information 018 PN_18.pdf (No. 18 (Feb. 28, 2016)) Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP ISSN 1348-1770 (online edition) Sapporo, Japan Psocid News The Psocidologists’ Newsletter No. 18 (Feb 28, 2016) Echmepteryx madagascariensis (Okinawa, Japan) ADDITIONS AND CORRECTIONS (PART 15) TO LIENHARD & SMITHERS, 2002: "PSOCOPTERA (INSECTA) – WORLD CATALOGUE AND BIBLIOGRAPHY" Charles LIENHARD (Geneva Natural History Museum, Switzerland) E-mail: [email protected] 1. Introduction This is the 15th part of a series of "Additions and Corrections to the World Catalogue and Bibliography" (Lienhard & Smithers, 2002) published in "Psocid News". Parts 1-14 were published in Psocid News no. 4-17 (see below); a Synthesis of Parts 1-10 is available online at the Geneva Museum's homepage: http://www.ville-ge.ch/mhng/psocoptera/divers/synthesis_add_1_10.pdf Please send me regularly copies of your papers on Psocoptera, and please inform me about errors that you find in Lienhard & Smithers (2002). If papers which came to your notice are not treated in the "Additions", please send me the bibliographical references by e-mail. In the "Additions to the Bibliography", references to the papers which I have not yet seen are marked with "(Not seen)" or "(Only abstract seen)". Please send me a copy or PDF of these papers if you feel concerned. -

(Diptera: Ephydridae), I: Revision of the Nearctic Species of Notiphila Fallen, Excluding the Caudata Group
Studies of Notiphilinae (Diptera: Ephydridae), I: Revision of the Nearctic Species of Notiphila Fallen, Excluding the caudata Group WAYNE N. MATHIS SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 287 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoo/ogy Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world cf science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -

Morphology of Psocomorpha (Psocodea: 'Psocoptera')
Title MORPHOLOGY OF PSOCOMORPHA (PSOCODEA: 'PSOCOPTERA') Author(s) Yoshizawa, Kazunori Insecta matsumurana. New series : journal of the Faculty of Agriculture Hokkaido University, series entomology, 62, 1- Citation 44 Issue Date 2005-12 Doc URL http://hdl.handle.net/2115/10524 Type bulletin (article) File Information Yoshizawa-62.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP INSECTA MATSUMURANA NEW SERIES 62: 1–44 DECEMBER 2005 MORPHOLOGY OF PSOCOMORPHA (PSOCODEA: 'PSOCOPTERA') By KAZUNORI YOSHIZAWA Abstract YOSHIZAWA, K. 2005. Morphology of Psocomorpha (Psocodea: 'Psocoptera'). Ins. matsum. n. s. 62: 1–44, 24 figs. Adult integumental morphology of the suborder Psocomorpha (Psocodea: 'Psocoptera') was examined, and homologies and transformation series of characters throughout the suborder and Psocoptera were discussed. These examinations formed the basis of the recent morphology-based cladistic analysis of the Psocomorpha (Yoshizawa, 2002, Zool. J. Linn. Soc. 136: 371–400). Author's address. Systematic Entomology, Graduate School of Agriculture, Hokkaido University, Sapporo, 060-8589 Japan. E-mail. [email protected]. 1 INTRODUCTION Psocoptera (psocids, booklice or barklice) are a paraphyletic assemblage of non-parasitic members of the order Psocodea (Lyal, 1985; Yoshizawa & Johnson, 2003, 2005; Johnson et al., 2004), containing about 5500 described species (Lienhard, 2003). They are about 1 to 10 mm in length and characterized by well-developed postclypeus, long antennae, pick-like lacinia, reduced prothorax, well-developed pterothorax, etc. Phylogenetically, Psocoptera compose a monophyletic group (the order Psocodea) with parasitic lice ('Phtiraptera': biting lice and sucking lice) (Lyal, 1985; Yoshizawa & Johnson, 2003, in press; Johnson et al., 2004). The order is related to Thysanoptera (thrips) and Hemiptera (bugs, cicadas, etc.) (Yoshizawa & Saigusa, 2001, 2003, but see also Yoshizawa & Johnson, 2005). -

Evolutionary Diversification of the Gall Midge Genus Asteromyia
Molecular Phylogenetics and Evolution 54 (2010) 194–210 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Evolutionary diversification of the gall midge genus Asteromyia (Cecidomyiidae) in a multitrophic ecological context John O. Stireman III a,*, Hilary Devlin a, Timothy G. Carr b, Patrick Abbot c a Department of Biological Sciences, Wright State University, 3640 Colonel Glenn Hwy., Dayton, OH 45435, USA b Department of Ecology and Evolutionary Biology, Cornell University, E145 Corson Hall, Ithaca, NY 14853, USA c Department of Biological Sciences, Vanderbilt University, Box 351634 Station B, Nashville, TN 37235, USA article info abstract Article history: Gall-forming insects provide ideal systems to analyze the evolution of host–parasite interactions and Received 3 April 2009 understand the ecological interactions that contribute to evolutionary diversification. Flies in the family Revised 17 August 2009 Cecidomyiidae represent the largest radiation of gall-forming insects and are characterized by complex Accepted 9 September 2009 trophic interactions with plants, fungal symbionts, and predators. We analyzed the phylogenetic history Available online 16 September 2009 and evolutionary associations of the North American cecidomyiid genus Asteromyia, which is engaged in a complex and perhaps co-evolving community of interactions with host-plants, fungi, and parasitoids. Keywords: Mitochondrial gene trees generally support current classifications, but reveal extensive cryptic diversity Adaptive diversification within the eight named species. Asteromyia likely radiated after their associated host-plants in the Aste- Fungal mutualism Insect-plant coevolution reae, but species groups exhibit strong associations with specific lineages of Astereae. Evolutionary asso- Cryptic species ciations with fungal mutualists are dynamic, however, and suggest rapid and perhaps coordinated Parasitoid changes across trophic levels. -

Proceedings of the United States National Museum
Proceedings of the United States National Museum SMITHSONIAN INSTITUTION . WASHINGTON, D.C. Volume 121 1967 Number 3569 SOLDIER FLY LARVAE IN AMERICA NORTH OF MEXICO ' By Max W. McFadden ^ The Stratiomyidae or soldier flies are represented in America north of Mexico by approximately 237 species distributed through 37 genera. Prior to this study, larvae have been described for only 21 species representmg 15 genera. In addition to the lack of adequate descriptions and keys, classification has seldom been attempted and a phylogenetic treatment of the larvae has never been presented. The present study has been undertaken with several goals in mind: to rear and describe (1) as many species as possible; (2) to redescribe all previously described larvae of North American species; and (3), on the basis of larval characters, to attempt to define various taxo- nomic units and show phylogenetic relationships withm the family and between it and other closely related familes. Any attempt to establish subfamilial and generic lunits must be regarded as tentative. This is especially true in the present study since larvae of so many species of Stratiomyidae remain unknown. Undoubtably, as more species are reared, changes mil have to be made in keys and definitions of taxa. The keys have been prepared chiefly for identification of last mstar larvae. If earher mstars are known, they either have been 1 Modified from a Ph. D. dissertation submitted to the University of Alberta E(hnonton, Canada. ' 2 Entomology Research Division, U.S. Dept. Agriculture, Tobacco Insects Investigations, P.O. Box 1011, Oxford, N.C. 27565. : 2 PROCEEDINGS OF THE NATIONAL MUSEUM vol.