Arthropod Community Structure in Regenerating Douglas-Fir and Red Alder Forests: Influences of Geography, Tree Diversity and Density
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ARTHROPOD COMMUNITIES and PASSERINE DIET: EFFECTS of SHRUB EXPANSION in WESTERN ALASKA by Molly Tankersley Mcdermott, B.A./B.S
Arthropod communities and passerine diet: effects of shrub expansion in Western Alaska Item Type Thesis Authors McDermott, Molly Tankersley Download date 26/09/2021 06:13:39 Link to Item http://hdl.handle.net/11122/7893 ARTHROPOD COMMUNITIES AND PASSERINE DIET: EFFECTS OF SHRUB EXPANSION IN WESTERN ALASKA By Molly Tankersley McDermott, B.A./B.S. A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Biological Sciences University of Alaska Fairbanks August 2017 APPROVED: Pat Doak, Committee Chair Greg Breed, Committee Member Colleen Handel, Committee Member Christa Mulder, Committee Member Kris Hundertmark, Chair Department o f Biology and Wildlife Paul Layer, Dean College o f Natural Science and Mathematics Michael Castellini, Dean of the Graduate School ABSTRACT Across the Arctic, taller woody shrubs, particularly willow (Salix spp.), birch (Betula spp.), and alder (Alnus spp.), have been expanding rapidly onto tundra. Changes in vegetation structure can alter the physical habitat structure, thermal environment, and food available to arthropods, which play an important role in the structure and functioning of Arctic ecosystems. Not only do they provide key ecosystem services such as pollination and nutrient cycling, they are an essential food source for migratory birds. In this study I examined the relationships between the abundance, diversity, and community composition of arthropods and the height and cover of several shrub species across a tundra-shrub gradient in northwestern Alaska. To characterize nestling diet of common passerines that occupy this gradient, I used next-generation sequencing of fecal matter. Willow cover was strongly and consistently associated with abundance and biomass of arthropods and significant shifts in arthropod community composition and diversity. -

Distribution Records of Aphids (Hemiptera: Phylloxeroidea, Aphidoidea) Associated with Main Forest-Forming Trees in Northern Europe
© Entomologica Fennica. 5 December 2012 Distribution records of aphids (Hemiptera: Phylloxeroidea, Aphidoidea) associated with main forest-forming trees in Northern Europe Andrey V. Stekolshchikov & Mikhail V. Kozlov Stekolshchikov, A. V.& Kozlov, M. V.2012: Distribution records of aphids (He- miptera: Phylloxeroidea, Aphidoidea) associated with main forest-forming trees in Northern Europe. — Entomol. Fennica 23: 206–214. We report records of 25 species of aphids collected from four species of woody plants (Pinus sylvestris, Picea abies, Betula pubescens and B. pendula)at50 study sites in Northern Europe, located from 59° to 70° N and from 10° to 60° E. Critical evaluation of earlier publications demonstrated that in spite of the obvi- ous limitations of our survey, the obtained information substantially contributed to the knowledge of the distribution of aphids in North European Russia, includ- ing Murmansk oblast (103 species recorded to date), Republic of Karelia (58 spe- cies), Arkhangelsk oblast (37 species), Vologda oblast (17 species) and Republic of Komi (29 species). We confirm the occurrence of Cinara nigritergi in South- ern Karelia; Pineus cembrae, Cinara pilosa and Monaphis antennata are for the first time recorded in Norway. A. V.Stekolshchikov, Zoological Institute, Russian Academy of Sciences, Univer- sitetskaya nab. 1, St. Petersburg 199034, Russia; E-mail: [email protected] M. V. Kozlov, Section of Ecology, University of Turku, FI-20014 Turku, Finland; E-mail: [email protected] Received 2 February 2012, accepted 5 April 2012 1. Introduction cades (e.g., Albrecht 2012), no comparable data (with rare exceptions) exist for the northern parts Species distributions and, consequently, spatial of the European Russia. -

DEFOLIATORS Insect Sections
Alaska. Reference is made to this map in selected DEFOLIATORS insect sections. (Precipitation information from Schwartz, F.K., and Miller, J.F. 1983. Probable maximum precipitation and snowmelt criteria for Fewer defoliator plots (27 plots) were visited during southeast Alaska: National Weather Service the 1999 aerial survey than in previous years (52 plots) Hydrometeorological Report No. 54. 115p. GIS layer throughout southeast Alaska. An effort was made to created by: Tim Brabets, 1997. distribute these plots evenly across the archipelago. URL:http://agdc.usgs.gov/data/usgs/water) The objectives during the 1999 season were to: ¨ Spend more time covering the landscape during Spruce Needle Aphid the aerial survey, Elatobium abietinum Walker ¨ Allow more time to land and identify unknown mortality and defoliation, and Spruce needle aphids feed on older needles of Sitka ¨ Avoid visit sites that were hard to get to and had spruce, often causing significant amounts of needle few western hemlocks. drop (defoliation). Defoliation by aphids cause reduced tree growth and can predispose the host to Hemlock sawfly and black-headed budworm larvae other mortality agents, such as the spruce beetle. counts were generally low in 1999 as they were in Severe cases of defoliation alone may result in tree 1998. The highest sawfly larvae counts were from the mortality. Spruce in urban settings and along marine plots in Thorne Bay and Kendrick Bay, Prince of shorelines are most seriously impacted. Spruce aphids Wales Island. Larval counts are used as a predictive feed primarily in the lower, innermost portions of tree tool for outbreaks of defoliators. For example, if the crowns, but may impact entire crowns during larval sample is substantially greater in 1999, then an outbreaks. -

Forest Insect and Disease Conditions in the United States 2000
United States Department Forest Insect and Of Agriculture Forest Service Disease Conditions Forest Health Protection in the United States March 2002 2000 Healthy Forests Make A World of Difference United States Department Of Agriculture Forest Insect and Forest Service Disease Conditions Forest Health Protection in the United States March 2002 2000 PREFACE This is the 50th annual report prepared by the U.S. • seed orchard insects and diseases; Department of Agriculture Forest Service (USDA • nursery insects and diseases; and Forest Service) of the insect and disease conditions of • abiotic damage. the Nation's forests. This report responds to direction in the Cooperative Forestry Assistance Act of 1978, as These categories are listed in the table of contents; amended, to conduct surveys and report annually on there is no index. insect and disease conditions of major national significance. Insect and disease conditions of local The information in this report is provided by the Forest importance are reported in regional and State reports. Health Protection Program of the USDA Forest Service. This program serves all Federal lands, The report describes the extent and nature of insect- including the National Forest System and the lands and disease-caused damage of national significance in administered by the Departments of Defense and 2000. As in the past, selected insect and disease Interior. Service is also provided to tribal lands. The conditions are highlighted in the front section of the program provides assistance to private landowners report. Maps are provided for some pests showing through the State foresters. A key part of the program affected counties in the East and affected areas in the is detecting and reporting insect and disease epidemics West. -

Evidence and Implications of Recent and Projected Climate Change in Alaska’S Forest Ecosystems 1, 2 1 3 4 JANE M
Evidence and implications of recent and projected climate change in Alaska’s forest ecosystems 1, 2 1 3 4 JANE M. WOLKEN, TERESA N. HOLLINGSWORTH, T. SCOTT RUPP, F. STUART CHAPIN, III, SARAH F. TRAINOR, 5 6 7 3 8 TARA M. BARRETT, PATRICK F. SULLIVAN, A. DAVID MCGUIRE, EUGENIE S. EUSKIRCHEN, PAUL E. HENNON, 9 10 11 8 1 ERIK A. BEEVER, JEFF S. CONN, LISA K. CRONE, DAVID V. D ’AMORE, NANCY FRESCO, 8 3 12 11 13 THOMAS A. HANLEY, KNUT KIELLAND, JAMES J. KRUSE, TRISTA PATTERSON, EDWARD A. G. SCHUUR, 14 14 DAVID L. VERBYLA, AND JOHN YARIE 1Scenarios Network for Alaska and Arctic Planning, University of Alaska, 3352 College Road, Fairbanks, Alaska 99709 USA 2United States Department of Agriculture Forest Service, Pacific Northwest Research Station, Boreal Ecology Cooperative Research Unit, P.O. Box 756780, University of Alaska, Fairbanks, Alaska 99775 USA 3Institute of Arctic Biology, University of Alaska, Fairbanks, Alaska 99775 USA 4Alaska Center for Climate Assessment and Policy, University of Alaska, 3352 College Road, Fairbanks, Alaska 99709 USA 5United States Department of Agriculture Forest Service, Pacific Northwest Research Station, Anchorage Forestry Sciences Laboratory, 3301 C Street, Suite 200, Anchorage, Alaska 99503 USA 6Environment and Natural Resources Institute, Department of Biological Sciences, University of Alaska, Anchorage, Alaska 99508 USA 7United States Geological Survey, Alaska Cooperative Fish and Wildlife Research Unit, University of Alaska, Fairbanks, Alaska 99775 USA 8United States Department of Agriculture Forest -

Alder Canopy Dieback and Damage in Western Oregon Riparian Ecosystems
Alder Canopy Dieback and Damage in Western Oregon Riparian Ecosystems Sims, L., Goheen, E., Kanaskie, A., & Hansen, E. (2015). Alder canopy dieback and damage in western Oregon riparian ecosystems. Northwest Science, 89(1), 34-46. doi:10.3955/046.089.0103 10.3955/046.089.0103 Northwest Scientific Association Version of Record http://cdss.library.oregonstate.edu/sa-termsofuse Laura Sims,1, 2 Department of Botany and Plant Pathology, Oregon State University, 1085 Cordley Hall, Corvallis, Oregon 97331 Ellen Goheen, USDA Forest Service, J. Herbert Stone Nursery, Central Point, Oregon 97502 Alan Kanaskie, Oregon Department of Forestry, 2600 State Street, Salem, Oregon 97310 and Everett Hansen, Department of Botany and Plant Pathology, 1085 Cordley Hall, Oregon State University, Corvallis, Oregon 97331 Alder Canopy Dieback and Damage in Western Oregon Riparian Ecosystems Abstract We gathered baseline data to assess alder tree damage in western Oregon riparian ecosystems. We sought to determine if Phytophthora-type cankers found in Europe or the pathogen Phytophthora alni subsp. alni, which represent a major threat to alder forests in the Pacific Northwest, were present in the study area. Damage was evaluated in 88 transects; information was recorded on damage type (pathogen, insect or wound) and damage location. We evaluated 1445 red alder (Alnus rubra), 682 white alder (Alnus rhombifolia) and 181 thinleaf alder (Alnus incana spp. tenuifolia) trees. We tested the correlation between canopy dieback and canker symptoms because canopy dieback is an important symptom of Phytophthora disease of alder in Europe. We calculated the odds that alder canopy dieback was associated with Phytophthora-type cankers or other biotic cankers. -

A Summary List of Fossil Spiders
A summary list of fossil spiders compiled by Jason A. Dunlop (Berlin), David Penney (Manchester) & Denise Jekel (Berlin) Suggested citation: Dunlop, J. A., Penney, D. & Jekel, D. 2010. A summary list of fossil spiders. In Platnick, N. I. (ed.) The world spider catalog, version 10.5. American Museum of Natural History, online at http://research.amnh.org/entomology/spiders/catalog/index.html Last udated: 10.12.2009 INTRODUCTION Fossil spiders have not been fully cataloged since Bonnet’s Bibliographia Araneorum and are not included in the current Catalog. Since Bonnet’s time there has been considerable progress in our understanding of the spider fossil record and numerous new taxa have been described. As part of a larger project to catalog the diversity of fossil arachnids and their relatives, our aim here is to offer a summary list of the known fossil spiders in their current systematic position; as a first step towards the eventual goal of combining fossil and Recent data within a single arachnological resource. To integrate our data as smoothly as possible with standards used for living spiders, our list follows the names and sequence of families adopted in the Catalog. For this reason some of the family groupings proposed in Wunderlich’s (2004, 2008) monographs of amber and copal spiders are not reflected here, and we encourage the reader to consult these studies for details and alternative opinions. Extinct families have been inserted in the position which we hope best reflects their probable affinities. Genus and species names were compiled from established lists and cross-referenced against the primary literature. -

Using Multiple Methodologies to Understand Within Species Variability of Adelges and Pineus (Hemiptera: Sternorrhyncha) Tav Aronowitz University of Vermont
University of Vermont ScholarWorks @ UVM Graduate College Dissertations and Theses Dissertations and Theses 2017 Using Multiple Methodologies to Understand within Species Variability of Adelges and Pineus (Hemiptera: Sternorrhyncha) Tav Aronowitz University of Vermont Follow this and additional works at: https://scholarworks.uvm.edu/graddis Part of the Biology Commons, Entomology Commons, and the Environmental Sciences Commons Recommended Citation Aronowitz, Tav, "Using Multiple Methodologies to Understand within Species Variability of Adelges and Pineus (Hemiptera: Sternorrhyncha)" (2017). Graduate College Dissertations and Theses. 713. https://scholarworks.uvm.edu/graddis/713 This Thesis is brought to you for free and open access by the Dissertations and Theses at ScholarWorks @ UVM. It has been accepted for inclusion in Graduate College Dissertations and Theses by an authorized administrator of ScholarWorks @ UVM. For more information, please contact [email protected]. USING MULTIPLE METHODOLOGIES TO UNDERSTAND WITHIN SPECIES VARIABILITY OF ADELGES AND PINEUS (HEMIPTERA: STERNORRHYNCHA) A Thesis Presented by Tav (Hanna) Aronowitz to The Faculty of the Graduate College of The University of Vermont In Partial Fulfillment of the Requirements for the Degree of Master of Science Specializing in Natural Resources May, 2017 Defense Date: March 6, 2016 Thesis Examination Committee: Kimberly Wallin, Ph.D., Advisor Ingi Agnarsson, Ph.D., Chairperson James D. Murdoch, Ph.D. Cynthia J. Forehand, Ph.D., Dean of the Graduate College ABSTRACT The species of two genera in Insecta: Hemiptera: Adelgidae were investigated through the lenses of genetics, morphology, life cycle and host species. The systematics are unclear due to complex life cycles, including multigenerational polymorphism, host switching and cyclical parthenogenesis. I studied the hemlock adelgids, including the nonnative invasive hemlock woolly adelgid on the east coast of the United States, that are currently viewed as a single species. -

Hemiptera: Adelgidae)
The ISME Journal (2012) 6, 384–396 & 2012 International Society for Microbial Ecology All rights reserved 1751-7362/12 www.nature.com/ismej ORIGINAL ARTICLE Bacteriocyte-associated gammaproteobacterial symbionts of the Adelges nordmannianae/piceae complex (Hemiptera: Adelgidae) Elena R Toenshoff1, Thomas Penz1, Thomas Narzt2, Astrid Collingro1, Stephan Schmitz-Esser1,3, Stefan Pfeiffer1, Waltraud Klepal2, Michael Wagner1, Thomas Weinmaier4, Thomas Rattei4 and Matthias Horn1 1Department of Microbial Ecology, University of Vienna, Vienna, Austria; 2Core Facility, Cell Imaging and Ultrastructure Research, University of Vienna, Vienna, Austria; 3Department of Veterinary Public Health and Food Science, Institute for Milk Hygiene, Milk Technology and Food Science, University of Veterinary Medicine Vienna, Vienna, Austria and 4Department of Computational Systems Biology, University of Vienna, Vienna, Austria Adelgids (Insecta: Hemiptera: Adelgidae) are known as severe pests of various conifers in North America, Canada, Europe and Asia. Here, we present the first molecular identification of bacteriocyte-associated symbionts in these plant sap-sucking insects. Three geographically distant populations of members of the Adelges nordmannianae/piceae complex, identified based on coI and ef1alpha gene sequences, were investigated. Electron and light microscopy revealed two morphologically different endosymbionts, coccoid or polymorphic, which are located in distinct bacteriocytes. Phylogenetic analyses of their 16S and 23S rRNA gene sequences assigned both symbionts to novel lineages within the Gammaproteobacteria sharing o92% 16S rRNA sequence similarity with each other and showing no close relationship with known symbionts of insects. Their identity and intracellular location were confirmed by fluorescence in situ hybridization, and the names ‘Candidatus Steffania adelgidicola’ and ‘Candidatus Ecksteinia adelgidicola’ are proposed for tentative classification. -
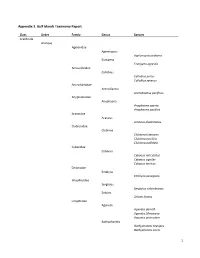
1 Appendix 3. Gulf Islands Taxonomy Report
Appendix 3. Gulf Islands Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis utahana Eratigena Eratigena agrestis Amaurobiidae Callobius Callobius pictus Callobius severus Antrodiaetidae Antrodiaetus Antrodiaetus pacificus Anyphaenidae Anyphaena Anyphaena aperta Anyphaena pacifica Araneidae Araneus Araneus diadematus Clubionidae Clubiona Clubiona lutescens Clubiona pacifica Clubiona pallidula Cybaeidae Cybaeus Cybaeus reticulatus Cybaeus signifer Cybaeus tetricus Dictynidae Emblyna Emblyna peragrata Gnaphosidae Sergiolus Sergiolus columbianus Zelotes Zelotes fratris Linyphiidae Agyneta Agyneta darrelli Agyneta fillmorana Agyneta protrudens Bathyphantes Bathyphantes brevipes Bathyphantes keeni 1 Centromerita Centromerita bicolor Ceratinops Ceratinops latus Entelecara Entelecara acuminata Erigone Erigone aletris Erigone arctica Erigone cristatopalpus Frederickus Frederickus coylei Grammonota Grammonota kincaidi Linyphantes Linyphantes nehalem Linyphantes nigrescens Linyphantes pacificus Linyphantes pualla Linyphantes victoria Mermessus Mermessus trilobatus Microlinyphia Microlinyphia dana Neriene Neriene digna Neriene litigiosa Oedothorax Oedothorax alascensis Pityohyphantes Pityohyphantes alticeps Pocadicnemis Pocadicnemis pumila Poeciloneta Poeciloneta fructuosa Saaristoa Saaristoa sammamish Scotinotylus Scotinotylus sp. 5GAB Semljicola Semljicola sp. 1GAB Sisicottus Spirembolus Spirembolus abnormis Spirembolus mundus Tachygyna Tachygyna ursina Tachygyna vancouverana Tapinocyba Tapinocyba -

Cooley Spruce Gall Adelgid
Cooley Spruce Gall Adelgid Mary Ridout, Graduate Student Plant Science 464, Fall 2007 Figure 1. Infested white spruce at the University of Idaho Plant Science Farm Cooley spruce gall adelgid damage was found on a White spruce (Picea glauca) at the University of Idaho Plant Science farm just outside Moscow, Idaho. The diagnosis was made from the observation of galls at or near the terminal ends of the branches (see Figure 1). Only new growth from the current year was affected. Galls were first observed early in the summer on terminal new growth as soft swollen tissue surrounding the needles. Some of these galls were green; others were beginning to turn a brownish purple. Figure 1 shows the appearance of the gall in late summer and fall. The plant itself was growing well, but damage from previous infestations was visible in slightly distorted growth caused by the loss of the terminal branch ends which induced lateral bud formation and branching and thereby formation of “shrubby” branches and asymmetrical growth habit. Spruce galls are caused by the Cooley spruce gall adelgid (Adelges cooleyi). A small aphid-like insect, the Cooley spruce gall adelgid alternates its life cycle between Picea species and Douglas Fir (Pseudotsuga menziesii). Galls, however, are only formed on spruce. While most commonly found on Colorado blue spruce, adelgids will also infest white, Engelmann, Norway, and Sitka spruce. Reproductive female adelgids over-winter on the undersides of the branches. When spring comes, they emerge and move to the new growth at the branch terminals where they lay their eggs at the base of the needles. -

Bees, Wasps & Ants
Sheringham and Beeston Regis Commons SSSI / SAC FAUNA: Hymenoptera INSECTA (Pterygota) Family/Order English Name. Scientific Name. Authority. Grid Ref. Tetrad/ Last Km sq. Common. Record. HYMENOPTERA. PAMPHILIDAE: Sawfly. Pamphilius inanitus (Villers, 1789) TG1642 1987? (Bees, Wasps and Ants) ARGIDAE: Elm Zig-zag Sawfly. Aproceros leucopoda Takeuchi, 1939 TG1642 14R/B 2020 Bramble Sawfly. Arge cyaneocrocea (Forster, 1771) TG1642 2016 Sawfly. Arge gracilicornis (Klug, 1814 ) TG1642 1987? CIMBICIDAE: Honeysuckle Sawfly. Abia lonicerae (Linnaeus) TG1641 14Q/B 2015 Club-horned Sawfly. Abia sericera (Linnaeus) TG1642 14R/B 2014 Club-horned Sawfly. Zaraea fasciata Linnaeus, 1758 TG1641/42 14R,14Q/B 2014 Birch Sawfly. Cimbex femoratus (Linnaeus, 1758) TG1642 14R/B 2017 SIRICIDAE: Greater Horntail Wasp. Urocerus gigas (Linnaeus, 1758) TG1642 14R/S 1992 CEPHIDAE: Sawfly. Calameuta pallipes (Klug, 1803) TG1642 1987? TENTHREDINIDAE: Willow Sawfly. Pontania proxima (Lepeletier, 1823) TG1642 14R/BS 2009 Willow Sawfly. Eupontania pedunculi (Hartig, 1837) TG1642 14R/B 1999 Willow Sawfly. Eupontainia viminalis (Linnaeus, 1758) TG1642 14R/B 2002 Willow Sawfly. Pontainia bridgemanii (Cameron, 1883) TG1642 14R/B 1999 Sawfly. Caliroa annulipes (Klug, 1816) TG1642 14R/S 2002 Hazel Sawfly. Craesus septentrionalis (Linnaeus, 1758) TG1641 14Q/B 2017 Sawfly. Blennocampa phyllocolpa Viitasaari & Vikberg, 1985 TG1642/41 14R,14Q/B 2003 Sawfly. Selandria serva (Fabricius, 1793) TG1642 14R/B 2013 Sawfly. Aneugmenus padi (Linnaeus, 1761) TG1642 1987? Bracken Sawfly. Strongylogaster multifasciata (Geoffroy, 1785) TG1642 14R/BS 2020 Sawfly. Dichrodolerus vestigialis (Klug, 1818) TG1642 1996 Sawfly. Dolerus germanicus (Fabricius, 1775) TG1642 1987? Sawfly. Eutomostethus ephippium (Panzer, 1798) TG1642 14R/BS 2020 Sawfly. Poodolerus aeneus Hartig, 1837 TG1642 1987? Sawfly. Dolerus brevitarus Hartig TG1642 1987? Sawfly.