Aerial and Aqueous Dispersion of Inorganic Contaminants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
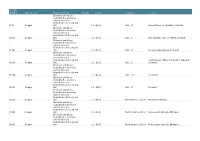
BRSUG Number Mineral Name Hey Index Group Hey No
BRSUG Number Mineral name Hey Index Group Hey No. Chem. Country Locality Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-37 Copper Au) 1.1 4[Cu] U.K., 17 Basset Mines, nr. Redruth, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-151 Copper Au) 1.1 4[Cu] U.K., 17 Phoenix mine, Cheese Wring, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-280 Copper Au) 1.1 4[Cu] U.K., 17 County Bridge Quarry, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and South Caradon Mine, 4 miles N of Liskeard, B-319 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-394 Copper Au) 1.1 4[Cu] U.K., 17 ? Cornwall? Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-395 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-539 Copper Au) 1.1 4[Cu] North America, U.S.A Houghton, Michigan Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-540 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-541 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, -

1850 Cornwall Quarter Sessions and Assizes
1850 Cornwall Quarter Sessions and Assizes Table of Contents 1. Epiphany Sessions ..................................................................................................................................... 1 2. Lent Assizes ............................................................................................................................................... 8 3. Easter Sessions ........................................................................................................................................ 46 4. Midsummer Sessions .............................................................................................................................. 54 5. Summer Assizes ....................................................................................................................................... 69 6. Michaelmas Sessions ............................................................................................................................... 93 Royal Cornwall Gazette 4 and 11 January 1850 1. Epiphany Sessions These Sessions were opened on Tuesday, the 1st of January, before the following magistrates:— J. KING LETHBRIDGE, Esq. Chairman; Sir W. L. S. Trelawny, Bart. E. Stephens, Esq. T. J. Agar Robartes, Esq., M.P. R. Gully Bennet, Esq. N. Kendall, Esq. T. H. J. Peter, Esq. W. Hext, Esq. H. Thomson, Esq. J. S. Enys, Esq. D. P. Hoblyn, Esq. J. Davies Gilbert, Esq. Revds. W. Molesworth, C. Prideaux Brune, Esq. R. G. Grylls, C. B. Graves Sawle, Esq. A. Tatham, W. Moorshead, Esq. T. Phillpotts, W. -

Camborne School of Mines CSMM143: Poldice Assignment James Heslington: 640012228
College of Engineering, Mathematics & Physical Sciences Camborne School of Mines CSMM143: Poldice Assignment James Heslington: 640012228 CSMM143: GIS for Surveyors Poldice Assignment 640012228 Contents Part A ..................................................................................................................................................... 3 1.1. Overview ........................................................................................................................................ 3 2.1. Site 1 Data ....................................................................................................................................... 4 2.2. Interpretation ................................................................................................................................................ 6 2.3. Spatial Recommendations ............................................................................................................................. 6 2.4. Recommendations ......................................................................................................................................... 6 3.1. Site 2 Data ...................................................................................................................................... 7 Part B .................................................................................................................................................... 11 Aim ..................................................................................................................................................... -

Cambridge University Press 978-1-108-47093-3 — Trace Metals in the Environment and Living Organisms Philip S
Cambridge University Press 978-1-108-47093-3 — Trace Metals in the Environment and Living Organisms Philip S. Rainbow Index More Information INDEX Aberdeenshire, 34, 156 Agrostis, 148, 160, 251, 271, 664 Ammodytes, 627 Aberllyn mine, 173 Merseyside refinery, 142, 166, 221, 249 Ampharete, 598 Aberystwyth, 172, 307, 370, 471, 498 tolerance, 172–173, 175, 268 ampharetid polychaetes, 598, See also Abington, 312 Aire River, 432 Ampharete, Melinna Abramis. See bream Aire valley, 32 Amphibalanus, 112, 495, 557 absorption, 68, See also uptake Airedale, 33, 54 Amphinemura, 343–344, 387 Acanthephyra, 585 ALAD, 116, 124, 222, 382, 547 amphipod crustaceans, 362, 551, See also acanthite, 29 Alca. See razorbill Corophium, Echinogammarus, Acarospora, 152, 155–156 alderflies, 318, 361, See also Sialis Gammarus, hyperiids, Rhepoxynius, Acartia, 551, 579–580 Alderley Edge, 27, 34–36 stegocephalids, talitrids Acaster, 432 alga, 286, 323, See also brown algae, bioaccumulation, 90, 99, 101, 103 acceptable daily input (ADI), 266, 660 green algae, phytoplankton, red biomarkers, 119 accumulation, 90 algae detoxification, 98, 101 accumulation patterns, 99 alginates, 441 uptake rates, 83, 85 Achillea, 163 Alice in Wonderland, 8 Anaconda, 50 Achnanthes, 321 Aliivibrio, 551 Analasmocephalus, 247 Achnanthidium, 321–322 Alitta, 443, 450, 515, 551 Anas. See mallard ducks acid mine drainage, 22, 29, 66, 288, 315, alkali disease, 20 Ancylus, 366 317, 389 Alle. See little auk Anglesey, 26, 33, 35, 47, 55, 306 Acid Mine Drainage Index (AMDI), 316 Allen River, 160 anglesite, 28, 33 acid volatile sulphide (AVS), 286, Allendales, 28, 32, 54, 309, See also Allen Anglo-Saxons, 36 315, 397 River, East Allen, West Allen Anguilla. -

Coast-To-Coast Walkers Trail.Pub
Mining Trails – The Coast-to-Coast Walkers Trail – Portreath to Devoran and Penpol – 16.74 miles ********************************************************************************************** Full Route Directions, with the emphasis on detours for walkers only, avoiding the cycle route ************************************************************************************** Portreath to Cambrose, on the official cycle trail – 2.10 miles Start at the furthest NE corner of Portreath Harbour at 65489/45483. Start on the Coast-to-Coast, passing storyboards ( Portreath Harbour, Tramroad and Incline ) and follow a granite marker ( Devoran 12 miles ) bearing L on a track and a road (Lighthouse Hill and Cliff Terrace) to the Portreath Arms Hotel ( B&B ) on your L. At 0.19 miles follow MT sign (Devoran 11 ) on Sunnyvale Road At 0.47 miles, the road continues as a tarmac lane through trees. At an MT post at 0.51 miles, the lane becomes a rough track, through a gap by a gate ( sign Cornish Way ). After 20 yards pass a granite plaque ( Duke of Leeds Hills ), the route continues as a track through woodland, with the Redruth road below on the R. At 0.60 miles the track becomes tarmac, continuing through woods. At 1.05 miles go through a gap by a gate, cross a road, and through another gap by a gate ( marker Tolticken Hill ). The track continues as tarmac, through a hunting gate at Bridge ( Bridge Inn below ). ( 1.27 miles ) The tarmac ends and a track continues, through a gate, still in woodland, ( marker Bridge at 1.30 miles ) At a fork at 1.34 miles go R (WM), now between high hedges . At 1.77 miles, looking R you get your first view to Carn Brea . -

The Boulton and Watt Archive and the Matthew Boulton Papers from Birmingham Central Library Part 13: Boulton & Watt Correspondence and Papers (MS 3147/3/286404)
INDUSTRIAL REVOLUTION: A DOCUMENTARY HISTORY Series One: The Boulton and Watt Archive and the Matthew Boulton Papers from Birmingham Central Library Part 13: Boulton & Watt Correspondence and Papers (MS 3147/3/286404) DETAILED LISTING REEL 231 3/336 Henry Williams, 17791783 (12 items) Henry Williams was an engine erector who mainly worked on engines in the Midlands. These included the Wren’s Nest Forge engine near West Bromwich, and the engines at Coalbrookdale, Ketley and Donnington Wood in Shropshire. The letters are addressed to Matthew Boulton, James Watt, Watt’s assistant William Playfair and the engine firm’s clerk, John Buchanan. 1. Letter. Henry Williams (Wren’s Nest) to James Watt (Soho). 28 Aug. 1779. Docketed “An experiment.” 2. Letter. Henry Williams (Wren’s Nest) to William Playfair (Soho). 23 Jan. 1780. 3. Letter. Henry Williams (Ketley) to William Playfair (Soho). 2 Apr. 1780. 4. Letter. Henry Williams (Ketley) to James Watt (Soho). 24 Apr. 1780. 5. Letter. Henry Williams (Ketley) to Matthew Boulton (Soho). 23 Aug. 1781. 6. Letter. Henry Williams (Ketley) to John Buchanan [Soho]. 5 Sep. 1781. The bottom half of the letter has been torn away. The back of the sheet has been used for calculations. 7. Letter. Henry Williams (Ketley) to John Buchanan (Soho). 21 Jan. 1782. 8a. Letter. Henry Williams (Coalbrookdale) to Matthew Boulton (Soho). 27 Mar. 1782. Enclosing (b) below. b. Letter. Dr. William Moore (Penryndee) to Henry Williams. 20 Mar. 1782. 9. Letter. Henry Williams (Coalbrookdale) to John Buchanan (Soho). 28 May 1782. Docketed “Balance of engine.” His progress with the Coalbrookdale engine. -

Cornish Mineral Reference Manual
Cornish Mineral Reference Manual Peter Golley and Richard Williams April 1995 First published 1995 by Endsleigh Publications in association with Cornish Hillside Publications © Endsleigh Publications 1995 ISBN 0 9519419 9 2 Endsleigh Publications Endsleigh House 50 Daniell Road Truro, Cornwall TR1 2DA England Printed in Great Britain by Short Run Press Ltd, Exeter. Introduction Cornwall's mining history stretches back 2,000 years; its mineralogy dates from comparatively recent times. In his Alphabetum Minerale (Truro, 1682) Becher wrote that he knew of no place on earth that surpassed Cornwall in the number and variety of its minerals. Hogg's 'Manual of Mineralogy' (Truro 1825) is subtitled 'in wich [sic] is shown how much Cornwall contributes to the illustration of the science', although the manual is not exclusively based on Cornish minerals. It was Garby (TRGSC, 1848) who was the first to offer a systematic list of Cornish species, with locations in his 'Catalogue of Minerals'. Garby was followed twenty-three years later by Collins' A Handbook to the Mineralogy of Cornwall and Devon' (1871; 1892 with addenda, the latter being reprinted by Bradford Barton of Truro in 1969). Collins followed this with a supplement in 1911. (JRIC Vol. xvii, pt.2.). Finally the torch was taken up by Robson in 1944 in the form of his 'Cornish Mineral Index' (TRGSC Vol. xvii), his amendments and additions were published in the same Transactions in 1952. All these sources are well known, but the next to appear is regrettably much less so. it would never the less be only just to mention Purser's 'Minerals and locations in S.W. -

Wheal Jane Minewater Study Environmental Appraisal And
WHEAL JANE MINEWATER STUDY ENVIRONMENTAL APPRAISAL AND TREATMENT STRATEGY f\lRA - SoJ VV> “f-4 '-f WHEAL JANE MINEWATER STUDY Environmental Appraisal and Treatment Strategy NRA National Rivers Authority South Western Region Knight Piesold RPA Kanthack House Risk & Policy Analysts Ltd Station Road Warren House, Beccles Road Ashford, Kent Loddon, Norfolk TN23 1PP NRJ4 6JL WHEAL JANE MINEWATER STUDY ENVIRONMENTAL APPRAISAL AND TREATMENT STRATEGY Contents EXECUTIVE SUMMARY 1. INTRODUCTION 2. BACKGROUND 3. THE RELEASE OF MINEWATER FROM WHEAL JANE 4. EXISTING TREATMENT SYSTEM 5. THE CURRENT SITUATION 6. HYDROLOGICAL MODELLING 7. DEVELOPMENT OF WATER QUALITY OBJECTIVES 8. LOCATION OF LONG TERM TREATMENT PLANT 9. PREVENTION & CONTROL OF DISCHARGES 10. PASSIVE TREATMENT TECHNOLOGY 11. ACTIVE TREATMENT TECHNOLOGY 12. SLUDGE DISPOSAL 13. ECONOMIC BENEFITS OF IMPROVEMENTS IN WATER QUALITY 14. TREATMENT OPTIONS Final Version NRA South Western Knight Piesold Wheal Jane Minewater Study Executive Summary Environmental Appraisal and Treatment Strategy EXECUTIVE SUMMARY INTRODUCTION Wheal Jane is an abandoned underground tin mine in Cornwall. After mine closure in 1991, underground pumping ceased, allowing groundwater levels to recover, releasing acidic metal laden minewater into the Camon River. The result was a highly visible and widely reported pollution incident extending into the Fal Estuary. In 1992 the NRA set up a project with the following objectives: - • Amelioration of the effects of the metal rich minewater from Wheal Jane on the Camon River and Fal Estuary. • Development of water quality objectives for the Camon River. • Research into the most appropriate and cost effective long term treatment strategies for achieving various water quality objectives. This report provides the basis for the NRA’s recommendations, to the DoE, on the long-term options for treating the Wheal Jane minewater. -

Matthew Boulton and the Soho Mint Numismatic Circular April 1983 Volume XCI Number 3 P 78
MATTHEW BOULTON AND THE SOHO MINT: COPPER TO CUSTOMER by SUE TUNGATE A thesis submitted to The University of Birmingham for the degree of DOCTOR OF PHILOSOPHY Department of Modern History College of Arts and Law The University of Birmingham October 2010 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT Matthew Boulton (1728-1809) is well known as an eighteenth-century industrialist, the founder of Soho Manufactory and the steam-engine business of Boulton and Watt. Less well known are his scientific and technical abilities in the field of metallurgy and coining, and his role in setting up the Soho Mint. The intention of this thesis is to focus on the coining activities of Matthew Boulton from 1787 until 1809, and to examine the key role he played in the modernisation of money. It is the result of an Arts and Humanities Research Council-funded collaboration with Birmingham Museum and Art Gallery, where, after examination of their extensive collection of coins, medals, tokens and dies produced at the Soho Mint, .research was used to produce a catalogue. -

Ashton & Germoe Circular
Ashton & Germoe Circular Time: Allow 3 hours. Difficulty: Moderate. 1 Length: 6 /2 miles. Location: Take the A394 5 Penzance-Helston Road 4 and at Ashton park near the Post Office. 3 Parking: Roadside parking is limited. Refreshments: At Ashton 2 you can eat at the Lion & Lamb or buy snacks at 6 the Post Office. In summer, Tresowes Farm serves cream teas. 7 8 1 9 10 11 1 Tresowes Pits 2 Balwest 3 Tregonning Hill 12 4 Mount Whistle 5 Boscreege 6 Lady Gwendolen Mine 7 Germoe Church 8 St Germoe’s Well 9 Wheal Grey China Clay pit 10 Wheal Grey 11 Whippers Lane 12 Lion and Lamb © Crown copyright. All rights reserved Kerrier District Council LA0783362001 This gentle walk explores the countryside between Ashton and Germoe. Stretched out along the main road, the countryside around Ashton was once a hive of industry, with quarry horses pulling carts laden with granite down from Tregonning Hill or bricks from Wheal Grey china clay pits. Tucked away in a sheltered valley, is Germoe village with its ancient church Ashton post office c1940. that has changed little over the centuries. 22 Start your walk from the middle of Ashton at the Post Office. Loosely translated the name Ashton means ‘the place of the ash tree’. Today the Post Office is the only shop left in the village. In the past there were at least 6 shops, including an undertaker and a blacksmith, as well as a school, a church and four chapels. One shop had a lending library and sold wallpaper, paint, paraffin, knitting wool, reels of cotton. -

Trees on Hedges in Cornwall
TREES ON HEDGES IN CORNWALL © Robin Menneer 2007. Revised 2019. History / timber and wood / expansion of tin mining / the tanning industry / woodland hedges / tenancy restrictions on hedge trees / 20th century hedge trees come and go / Hooper's dubious "rule" / coppicing / veteran trees / restrictions on cutting down trees / planting trees on hedges / tree species in Cornish hedges / the future for Cornish hedge trees. I'd as soon think to see a cow climb a scaw-tree (Cornish expression of disbelief. Scaw - elder.) The importance of trees in hedges is inevitably linked with the availability of trees from woodland. Cornwall emerged from the last Ice Age with trees able to tolerate relatively cold conditions, notably birch and willow and, later, pine. As the climate warmed up, broad-leaved trees spread in. This is confirmed by the pollen found by archaeologist George Smith underneath the Bronze Age cairn at Chysauster, in West Penwith. Here pollen grains of birch, pine, elm, oak, lime, alder, yew, willow and hazel were recorded. Buried in the peat in the valley nearby, the same tree species were identified, plus beech, ash, holly and rowan. As the climate changed, so did the distribution of the different tree species. Our native woodland was never the dank dark covering of the modern close-planted commercial trees. Sunny glades have been an ever- present feature of the natural succession of trees, and prehistoric felling was an enlargement of these woodland clearings. The initial process of deforestation was begun by Sunny glades originally were enlarged into fields. Today most Mesolithic hunters perhaps as early as of Cornwall's trees are in the hedges. -

INDUSTRIAL REVOLUTION: a DOCUMENTARY HISTORY Series One: the Boulton and Watt Archive and the Matthew Boulton P
INDUSTRIAL REVOLUTION: A DOCUMENTARY HISTORY Series One: The Boulton and Watt Archive and the Matthew Boulton Papers from Birmingham Central Library Part 13: Boulton & Watt Correspondence and Papers (MS 3147/3/286404) DETAILED LISTING REEL 238 3/371 General Correspondence, B (63 items) 1. Letter. John Baddeley (Hanley) to James Watt [?]. 8 Sep. 1783. His prices for ironwork, woodwork and movements for a lathe. 2. Letter. George Bailey (London) to Matthew Boulton (Soho). 30 Jan. 1782. Details of his new flour mill. What size of engine will he need, what quantity of coal will it burn and how much will it cost. 3. Letter. R. Bakewell (Gresley Hall, near Burtonon Trent) to Boulton & Watt [Soho]. 26 Aug. 1779. Hopes the engine Boulton & Watt mentioned will be sufficient. Has sent for a man from Nottingham who he will send on to Soho for Boulton & Watt to instruct. 4. Letter. R. Bakewell (Gresley Hall, near Burtonon Trent) to James Watt [Birmingham]. 3 Sep. 1779. Details of his plans for the engine for Newhall Colliery. 5. Letter. R. Bakewell (Gresley Hall, near Burtonon Trent) to James Watt [Birmingham]. 7 Sep. 1779. Did not manage to send a letter to Logan Henderson and did not see Mr. Smith the engineer. Is now determined to proceed no further with the business until he has “recovered [himself] a little”. 6. Letter. John Baldwyn (Chepstow) to Boulton & Watt (Birmingham). 28 Aug. 1779. His goods have not yet arrived. Has heard from Mr. Wilkinson that the Mary is on her way from Chester. Can Boulton & Watt arrange for the goods to be forwarded.