Pattern and Determinants of Nutritional Status Among Children Attending Primary Schools in a Rural and an Urban Community in Osun State; a Comparative Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
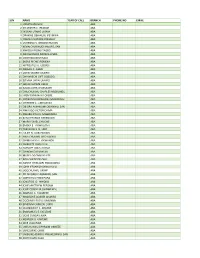
PROVISIONAL LIST.Pdf
S/N NAME YEAR OF CALL BRANCH PHONE NO EMAIL 1 JONATHAN FELIX ABA 2 SYLVESTER C. IFEAKOR ABA 3 NSIKAK UTANG IJIOMA ABA 4 ORAKWE OBIANUJU IFEYINWA ABA 5 OGUNJI CHIDOZIE KINGSLEY ABA 6 UCHENNA V. OBODOCHUKWU ABA 7 KEVIN CHUKWUDI NWUFO, SAN ABA 8 NWOGU IFIONU TAGBO ABA 9 ANIAWONWA NJIDEKA LINDA ABA 10 UKOH NDUDIM ISAAC ABA 11 EKENE RICHIE IREMEKA ABA 12 HIPPOLITUS U. UDENSI ABA 13 ABIGAIL C. AGBAI ABA 14 UKPAI OKORIE UKAIRO ABA 15 ONYINYECHI GIFT OGBODO ABA 16 EZINMA UKPAI UKAIRO ABA 17 GRACE UZOME UKEJE ABA 18 AJUGA JOHN ONWUKWE ABA 19 ONUCHUKWU CHARLES NSOBUNDU ABA 20 IREM ENYINNAYA OKERE ABA 21 ONYEKACHI OKWUOSA MUKOSOLU ABA 22 CHINYERE C. UMEOJIAKA ABA 23 OBIORA AKINWUMI OBIANWU, SAN ABA 24 NWAUGO VICTOR CHIMA ABA 25 NWABUIKWU K. MGBEMENA ABA 26 KANU FRANCIS ONYEBUCHI ABA 27 MARK ISRAEL CHIJIOKE ABA 28 EMEKA E. AGWULONU ABA 29 TREASURE E. N. UDO ABA 30 JULIET N. UDECHUKWU ABA 31 AWA CHUKWU IKECHUKWU ABA 32 CHIMUANYA V. OKWANDU ABA 33 CHIBUEZE OWUALAH ABA 34 AMANZE LINUS ALOMA ABA 35 CHINONSO ONONUJU ABA 36 MABEL OGONNAYA EZE ABA 37 BOB CHIEDOZIE OGU ABA 38 DANDY CHIMAOBI NWOKONNA ABA 39 JOHN IFEANYICHUKWU KALU ABA 40 UGOCHUKWU UKIWE ABA 41 FELIX EGBULE AGBARIRI, SAN ABA 42 OMENIHU CHINWEUBA ABA 43 IGNATIUS O. NWOKO ABA 44 ICHIE MATTHEW EKEOMA ABA 45 ICHIE CORDELIA CHINWENDU ABA 46 NNAMDI G. NWABEKE ABA 47 NNAOCHIE ADAOBI ANANSO ABA 48 OGOJIAKU RUFUS UMUNNA ABA 49 EPHRAIM CHINEDU DURU ABA 50 UGONWANYI S. AHAIWE ABA 51 EMMANUEL E. -

Finance-And-Revenue-Mobilization
FINANCE & REVENUE MOBILIZATION SECTOR 2020 – 2022 MEDIUM-TERM SECTOR STRATEGY (MTSS) JULY, 2019 TABLE OF CONTENT Pages Foreword ii-iii Acknowledgements iv List of Tables v Executive Summary 1-2 1 CHAPTER ONE – OBJECTIVES OF THE DOCUMENT 1.1 Objectives of the MTSS Document 3 1.2 Outline of the Structure of the Document 3-4 1.3 Process used for the MTSS Development 4 1.4 Summary of the sector’s Programmes, Outcomes and Related Expenditures 5 2 CHAPTER TWO – THE SECTOR AND POLICY IN THE STATE 2.1 A Brief Introduction to the State 6-8 2.2 Overview of the Finance and Revenue Mobilization Sector 8 2.3 The Current Situation in the Sector 9 2.4 Sector Policy 10 2.5 The Sector’s, Mission, Vision and Core Values 10-11 2.6 The Sector’s Objectives and Programmes for the MTSS Period 11-14 3 CHAPTER THREE – THE DEVELOPMENT OF SECTOR STRATEGY 3.1 Major Strategic Challenges 15 3.2 Resource Constraints 16 3.3 Projects Prioritization 17-18 3.4 Personnel and Overhead Costs: Existing and Projections 19 3.5 Contributions from our Partners 19 3.6 Cross-Cutting Issues 19 3.7 Outline of Key Strategies 20-21 3.8 Justification 22 3.9 Responsibilities and Operational Plan 22-23 4 CHAPTER FOUR – THREE-YEAR EXPENDITURE PROJECTIONS 4.1 The process used to make Expenditure Projections 24 4.2 Outline Expenditure Projections 24 5 CHAPTER FIVE – MONITORING AND EVALUATION 5.1 Conducting Annual Sector Review 25 5.2 Organizational Arrangements 25-26 i FOREWORD The imperative of effective revenue mobilisation, generation and allocation cannot be over-emphasised. -

Sovereign-Trust-Insurance-2011-Annual-Report.Pdf
1 A N N U A L R E P O R T & A C C O U N T S 2 0 1 1 V I S I O N To be a leading brand providing insurance and financial services of global standards. M I S S I O N To enhance the every day life of our customers t h ro u g h i n n o v a t i v e insurance and financial services while creating exceptional value for our shareholders. CORE VALUES Superior Customer Service Innovation Professionalism Integrity Empathy Team Spirit. A N N U A L R E A C C O P O R T U N T S & 2 2 0 1 1 Notice of AGM 03 Corporate Information 05 Management Team 07 Financial Highlights 08 Chairman’s Statement 09 Board of Directors 14 Management Team 18 Directors’ Report 24 Statement of Directors' Responsibilities 31 Independent Auditor’s Report 32 C O N T E N T S Report of the Audit Committee 33 Statement of Significant Accounting Policies 34 Balance Sheet 40 Profit and Loss Account 41 Revenue Account 42 Statement of Cash Flow 43 Notes to the Financial Statements 45 Statement of Value Added 63 Five-Year Financial Summary 64 Share Capital History 65 Mandate Form 66 Proxy Form 67 Admission Form 68 Unclaimed Dividend Warrant List 69 3 A N N U A L R E P O R T & A C C O U N T S 2 0 1 1 1 7 T H A G M N O T I C E 4 A N N U A L R E P O R T & A C C O U N T S 2 0 1 1 N O T I C E O F T H E 1 7 T H A N N U A L G E N E R A L M E E T I N G TO ALL THE SHAREHOLDERS NOTICE OF ANNUAL GENERAL NOTES CLOSURE OF REGISTER MEETING PROXIES The Register of members and Only a member of the Company Transfer Books of the Company shall NOTICE IS HEREBY GIVEN to you entitled to attend and vote at the be closed from 24th day of May that the 17th Annual General General Meeting is entitled to 2012 to 31st day of May 2012 (both Meeting of Sovereign Trust appoint a proxy in his/her stead. -

Final List of Delegations
Supplément au Compte rendu provisoire (21 juin 2019) LISTE FINALE DES DÉLÉGATIONS Conférence internationale du Travail 108e session, Genève Supplement to the Provisional Record (21 June 2019) FINAL LIST OF DELEGATIONS International Labour Conference 108th Session, Geneva Suplemento de Actas Provisionales (21 de junio de 2019) LISTA FINAL DE DELEGACIONES Conferencia Internacional del Trabajo 108.ª reunión, Ginebra 2019 La liste des délégations est présentée sous une forme trilingue. Elle contient d’abord les délégations des Etats membres de l’Organisation représentées à la Conférence dans l’ordre alphabétique selon le nom en français des Etats. Figurent ensuite les représentants des observateurs, des organisations intergouvernementales et des organisations internationales non gouvernementales invitées à la Conférence. Les noms des pays ou des organisations sont donnés en français, en anglais et en espagnol. Toute autre information (titres et fonctions des participants) est indiquée dans une seule de ces langues: celle choisie par le pays ou l’organisation pour ses communications officielles avec l’OIT. Les noms, titres et qualités figurant dans la liste finale des délégations correspondent aux indications fournies dans les pouvoirs officiels reçus au jeudi 20 juin 2019 à 17H00. The list of delegations is presented in trilingual form. It contains the delegations of ILO member States represented at the Conference in the French alphabetical order, followed by the representatives of the observers, intergovernmental organizations and international non- governmental organizations invited to the Conference. The names of the countries and organizations are given in French, English and Spanish. Any other information (titles and functions of participants) is given in only one of these languages: the one chosen by the country or organization for their official communications with the ILO. -

Voting Behaviour in Osun 2014 Governorship Election in Nigeria
Public Policy and Administration Research www.iiste.org ISSN 2224-5731(Paper) ISSN 2225-0972(Online) Vol.4, No.8, 2014 Voting Behaviour in Osun 2014 Governorship Election in Nigeria Adeolu Durotoye, PhD Department of Political Science and International Studies, College of Social and Management Sciences Afe Babalola University, Ado Ekiti, Ekiti State, Nigeria Email: [email protected], [email protected] Abstract The August 9, 2014 governorship election in Osun state, South West Nigeria was another test run for the Independent Electoral Commission (INEC) towards the 2015 General election. The election was important because it occurred not long after the Ekiti state governorship election of June 21, 2014 which was largely adjudged to be free and fair. Besides, the People’s Democratic Party (PDP) candidate won the Ekiti election by defeating the All Progressive Congress (APC) candidate who was the incumbent. The Ekiti election increased the momentum in Osun as the PDP was emboldened that it could pull the same feat in Osun, while the APC was hell bent on avoiding another slip.At the end of the day, the APC won in Osun and the PDP lost. What was responsible for this outcome? Why was the PDP not able to repeat the feat it achieved in Ekiti? These are the questions facing this paper. This paper will blend the unique aspects of the Osun election with a more general understanding of electoral behaviour to create a full explanation. Keywords : Voting behaviour, Election, Nigeria, Osun state, INEC, PDP, APC, Aregbesola, Omisore 1. Introduction The August 9, 2014 governorship election in Osun state, South West Nigeria was another test run for the Independent Electoral Commission (INEC) towards the 2015 General election. -

Mise En Page 1
The Africa Experts Ecobank Nigeria Plc Annual Report RC No: 89773 2010 15:27 The Ecobank Network 2 Ecobank Nigeria Annual Report 2010 Ecobank Nigeria branches Head Office Branch Plot 21, Ahmadu Bello Way - P.O. Box 72688, Victoria Island - Lagos - NIGERIA Tel: (234) 1 2710391/5 - Fax: (228) 221 51 19 CROSS RIVER / AKWA IBOM Abak Netpost Ogboefere Market NO 1 Ikot Ekpene Road Abak Tel.: (234) 806 099 7484/704 145 0365; CROSS RIVER STATE Tel.: (234) 07041450461 (234) 803 387 1772/704 145 0254 Old Market Road Calabar II EAST 24 Old Market Road, Onitsha 15, Murtala Mohammed Highway, Tel.: (234) 704 145 0386/704 145 0171 Calabar ABIA STATE Williams Tel.: (234) 803 720 4656/805 839 9981 7 William Street, Onitsha Mary Slessor Factory Road, Aba. Tel.: (234) 704 145 0366/803 335 7444; 12 Mary Slessor Avenue, Calabar No. 1, Factory Road, Aba. (234) 704 145 0907 Tel.: (234) 87 290 474/704 145 0474 Tel.: (234) 07041450398/07041450400 Obudu Ekeoha ENUGU STATE No. 2 Government Station Ranch Road, Ekeoha Shopping Complex, Obudu Ehi/Asa Road, Aba Okpara Avenue I Tel.: (234) 708 987 7826/803 418 6012 Tel.: (234) 704 145 0389; 704 145 0415 31A Okpara Avenue, Enugu Ogoja Govt. Station Layout, Umuahia Tel.: 234) 704 145 0381/704 145 0170; 19 MLA Hospital Road Igoli Ogoja Plot 110 Govt. Station Layout, Umuahia (234) 42 290 578/704 145 0380 Tel.: (234) 08037134913/08028363500 Tel.: (234) 704 145 0411/704 145 0225 Okpara Avenue II Ikom Faulks Road Aba 20B Okpara Avenue, Enugu 72 Calabar Road, Four Corner, Ikom 187 FAULKS ROAD,ABA ABIA-STATE Tel.: (234) 704 145 -

Nigerian Bar Association
NIGERIAN BAR ASSOCIATION COVID-19 PALLIATIVE DISBURSEMENT REPORT AS AT 27 AUGUST 2020 NIGERIAN BAR ASSOCIATION COVID-19 PALLIATIVE DISBURSEMENT REPORT Note NGN Receipt Donations 1 101,893,495 Less: Disbursements 2 85,680,000 Bank Charges 183,787 85,863,787 Available funds 16,029,708 *A total of 8,568 beneficiaries were paid out of the 8,610 valid applications received. The remaining 42 applicants are having issues with their bank accounts at the time of preparing this report. The names of 42 eligible applicants with bank issues have been disclosed in the note 3 of this report. 2 NIGERIAN BAR ASSOCIATION 1 SCHEDULE OF COVID-19 RELIEF DONATIONS RECEIVED S/N DONOR NGN 1 Godwin Omoaka SAN 100,000.00 2 Y C Maikyau SAN 500,000.00 3 Y C Maikyau & Co: Raphael Terfa Agbanyi 20,000.00 Obasi-Nwabueze 20,000.00 Tobechukwu Anthonia Rapu 20,000.00 Mohammed Adelodun 20,000.00 Pius Agbo Joseph 20,000.00 Abdulrauf Tijani Aboki 15,000.00 Ogochi Peace Nwosu 15,000.00 Oluwabusola Susan Oketade 15,000.00 Abdulrahman IsaWasegu 10,000.00 Faizah Danlami Zama 10,000.00 Abdulrahman Belgore 10,000.00 Chika Promise Nwozor 10,000.00 Nuratu Umar 10,000.00 Faisal Abubakar 10,000.00 Rachel David Mann 10,000.00 Firdausi Abdulahi Ahmad 10,000.00 Naomi Samariya Banku (NYSC-Member) 10,000.00 Kelechi Ogbonna 10,000.00 Anthony Chigozi Ekwoaba 6,000.00 Hannatu Ayuba Matunji 5,000.00 Morris Chijoke Duru 5,000.00 Hannah Charles Dikki 5,000.00 Belgore Mahmoud 5,000.00 Muhammad Muhammad Gajo 5,000.00 Nana Fatima Usman 5,000.00 Mary Omoyeme Musa 5,000.00 Yakubu Bako (Student) 5,000.00 4 Okocha & Okocha 500,000.00 5 Enitan Olukayode Abayomi SAN 100,000.00 6 Ayodele Akintunde & Co 250,000.00 7 Mrs Funke Adekoya SAN 250,000.00 8 Synergy Attornies/Abiodun Owonikoko SAN 250,000.00 9 Adegboyega Awomolo & Associates 500,000.00 10 Oluwafemi Peter Atoyebi SAN 1,000,000.00 11 Ogunwumiju Kehinde Olamide SAN 250,000.00 12 Adeniji Kazeem & Co 250,000.00 13 Dr. -
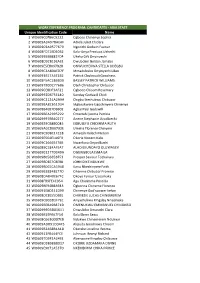
ABIA STATE Unique Identification Code Name 1
WORK EXPERIENCE PROGRAM: CANDIDATES - ABIA STATE Unique Identification Code Name 1 WO609C0FB6C9222 Ogboso Chinenye Sophia 2 WO609A3A978AEAF Adiele Juliet Chidera 3 WO609C9A9577679 Ngozichi Godwin Favour 4 WO608FD71ED50B2 Kalu-Anya Precious Uchechi 5 WO60995BB882FD4 Ukeka Gift Onyinyechi 6 WO608D92B19EAEE Owubokiri Golden Jamabo 7 WO609E5E8097B28 ONWUCHEKWA STELLA UDEGBU 8 WO609C3A80AED7F Mmadubuko Onyinyechi Lilian 9 WO6093B17A6E1B2 Patrick Chukwudi Goodness 10 WO608F5AC186BD9 BASSEY PATRICK WILLIAMS 11 WO609790DC776B6 Oteh Christopher Chibuzor 12 WO609CEBDF3AF21 Ogbodo Chisom Rosemary 13 WO60992D675E140 Sunday Godswill Chidi 14 WO609C2124A2A9A Chigbu Ikechukwu Chibuzor 15 WO609BA8C651934 Mgbeokwere Ugochinyere Chinenye 16 WO608B4E87DBB0E Agbai Paul Godswill 17 WO609B6A2995222 Omattah Ijeoma Patricia 18 WO609997BB40277 Anene Stephanie Uzodibechi 19 WO60929CB8B0045 EGBULEFU CHIDINMA RUTH 20 WO609A0CD00792E Umeha Florence Chinyere 21 WO609C9DBC1F228 Amaeshi Kelechi Nelson 22 WO609E564E1A6F9 Okorie Vincent Kalu 23 WO609C16665E7BB Nwankwo Onyedikachi 24 WO6089C184AFEA7 ALAO EKUNDAYO OLUWASEYI 25 WO60901277D349A OGUNGBOLA ISMAILA 26 WO609B05665B7F3 Prosper Saviour Tochukwu 27 WO609BDEE7CBEB8 JOHN DIKE NDUKWE 28 WO609B065CA5948 Kanu Nkechinyere Faith 29 WO609B18848E77D Ohanme Chibuzor Promise 30 WO608DAB40E67FC Okoye Favour Uzoamaka 31 WO608FB9FE4C954 Agu Chidimma Priscillia 32 WO609BE94B8A943 Ogbonna Chineme Florence 33 WO6091E8D241D99 Chinenye God'spower kelvin 34 WO608DCEE55DBB1 CHIMEZIE LUCAS CHINEMEREM 35 WO609CBD3D1F7EC Anyachukwu Kingsley -

Sec Quartely Publication 3Rd Quarter, 2012
1 SEC QUARTELY PUBLICATION 3RD QUARTER, 2012 2 TABLE OF CONTENT S/N DEPARTMENTS 02 PRIMARY AND SECONDARY MARKETS 15 MONITORING AND INVESTIGATION (MI) 28 SECURITIES AND INVESTMENTS SERVICES (SIS) 39 FINANCIAL STANDARD AND CORPORATE GOVERNANCE (FS&CG) 41 COLLECTIVE INVESTMENT SCHEMES (CIS) 51 LEGAL 61 ENFORCEMENT AND COMPLIANCE (E&C) 62 REGISTRATION AND RECOGNISED INVESTMENT EXCHANGES (RRIE) ACTIVITIES OF ZONAL OFFICES 68 IBADAN 70 KADUNA 71 KANO 73 ONITSHA 79 PORT HARCOURT 81 LAGOS 3 PRIMARY AND SECONDARY MARKET NEW ISSUES Just like the two preceeding quarters, the new issues floated in the third quarter of 2012 were dominantly Federal Government of Nigeria (FGN) bonds. Out of it, ten (10) new issues were recorded in the quarter, eight (8) were FGN bonds, accounting for about 96% (N210 billion) of the total value of issues which stood at N219.12 billion. The two other issues floated were equities valued at N9.12 billion. TABLE 1: SUMMARY OF NEW ISSUES IN THE THIRD QUARTER OF 2012 Mode of Offer No. of Issues Value of Shares(N’ m) Rights 1 5,523.09 Special Placement 1 3,600.00 Total Equities 2 9,123.09 Corporate Bond 0 0.00 Sub-national Bond 0 0.00 FGN Bonds 8 210,000.00 Total Debt 8 210,000.00 Overall 10 219,123.09 Source: SEC, DMO The trend in new issues so far in 2012 showed investor preference for FGN bonds due to favorable returns placed on the bonds. This had however made bond issuance very expensive to other issuers (State Governments and Companies) that had to benchmark against FGN bonds in addition to interest rate and inflation to attract investors. -

Writ of Summons
WRIT OF SUMMONS: On this day of two thousand and seventeen, at the request of (1) Mrs Esther Duke Kiobel, of Dallas, United States of America (2) Mrs Victoria Bera, of Winnipeg, Canada, (3) Mrs Blessing Ken Nordu, of Giokoo, Gokana, Nigeria and (4) Mrs Charity Vureka Levula, of Bomu, Gokana, Nigeria, all choosing their address for service in this matter at Linnaeusstraat 2A in Amsterdam (1092 CK) at the offices of Prakken d’Oliveira, Human Rights Lawyers, of which firm Ms. Ch. Samkalden and Mr. T. de Boer are handling this case and acting as counsel; I, With the proviso that the exhibits will not be served together with the summons, but will be submitted into the proceedings in a timely fashion, have summoned: (1) the legal entity under foreign law Royal Dutch Shell, plc, with its registered office in England and Wales in accordance with the articles of association and its registered office in The Hague, at Carel van Bylandtlaan 30 (2596 HR), serving my writ there at its office address and leaving a copy of this writ with: (2) the public company Shell Petroleum N.V., with registered office in The Hague, a successor by universal title of the public company N.V. Koninklijke Nederlandsche Petroleum Maatschappij, with registered office in The Hague, at Carel van Bylandtlaan 30 (2596 HR), serving my writ there at its office address and leaving a copy with: 1 (3) the legal entity under foreign law the Shell Transport and Trading Company, limited, with its registered office in London, United Kingdom and its principal place of business at the -

Osun State Pocket Fact Finder
HISTORICAL DEVELOPMENT: Osun, state, western Nigeria. Osun state was created in 1991 from the eastern third of Oyo state. It is bounded by the states of Kwara on the northeast, Ekiti and Ondo on the east, Ogun on the south, and Oyo on the west and northwest. The Yoruba Hills run through the northern part of Osun state. The state has a covering of tropical rain forest, and the Oshun is the most important river. Osun state is inhabited mainly by the Yoruba people. Osun’s economy is based mainly on agriculture. Major crops include yams, cassava (manioc), corn (maize), beans, millet, plantains, cacao, palm oil and kernels, and fruits. Cottage industries produce brass work, woven cloth, and wood carvings. Oshogbo, the state capital, has a textile industry, a food-processing plant, and a steel- rolling mill. The state’s tourist attractions include the Mbari Arts Centre at Oshogbo, the residential palaces of Yoruba rulers in Ilesha and Ile-Ife, and the Osun-Osogbo Sacred Grove, a forest that contains several shrines and artwork in honour of the Yoruba deity Osun (designated a UNESCO World Heritage site in 2005). The Obafemi Awolowo University (founded in 1961) is at Ile-Ife. Oshogbo is linked by road and railway to Ibadan in Oyo state. Pop. (2006) 3,423,535. GEOGRAPHY Osun State, Nigeria, came into existence on 27th August, 1991. The State which is one of the then nine newly created States was carved out of the old Oyo State by the General Ibrahim Babangida‘s administration. It is one of the 36 States w hich make up Nigeria. -

Springs Eternal
Hope Chams PLC springs eternal 2011 Annual Report and Accounts CHAMS PLC 2011 Annual Report Table of Contents Table of Contents 3 Consolidated Profit And Loss Account 27 Vision & Mission Statement 5 Consolidated Balance Sheet 28 Directors, Officers & Professional Consolidated Statement Advisers 7 of Cash Flows 29 Chairman’s Statement 9 Notes to the Financial Statements 30 A Brighter Tomorrow! 10 Consolidated Statement of Value Added 43 Corporate Governance 12 Consolidated Financial Summary 44 Directors’ Report 14 Products and Services 45 Report of the Audit committee 22 Subsidiaries 46 Report of the Independent Auditors 23 List of Unclaimed Dividends 47 Statement Of Significant Accounting Policies 24 Corporate Directory 55 At A Glance 26 Hope springs eternal 4 CHAMS PLC 2011 Annual Report Vision & Mission Statement OUR ENVISIONED FUTURE A company of Nigerian origin, we will be extremely influential and highly visible in global technol- ogy. Our trusted cutting edge technology will enhance the quality of life in a manner that glorifies God. We would be employer of first choice. OUR VISION To be the leading provider of innovative and beneficial technologies that improves the quality of life. OUR MISSION To design and deploy innovative and beneficial technologies, while creating value for all our stakeholders OUR CORE VALUES 1. Create a healthy and dynamic work environment characterized by Z-CASE • Zero tolerance for excuses • Candor • An entrepreneurial spirit, • Strong bonds between past and present Chams family • Excitement 2. It is imperative to delight our customers by making our technologies available 24/7 3. We commit to giving back to society through group activities that will positively impact on our communities.