Architecture of the Short External Rotator Muscles of the Hip
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hip Extensor Mechanics and the Evolution of Walking and Climbing Capabilities in Humans, Apes, and Fossil Hominins
Hip extensor mechanics and the evolution of walking and climbing capabilities in humans, apes, and fossil hominins Elaine E. Kozmaa,b,1, Nicole M. Webba,b,c, William E. H. Harcourt-Smitha,b,c,d, David A. Raichlene, Kristiaan D’Aoûtf,g, Mary H. Brownh, Emma M. Finestonea,b, Stephen R. Rossh, Peter Aertsg, and Herman Pontzera,b,i,j,1 aGraduate Center, City University of New York, New York, NY 10016; bNew York Consortium in Evolutionary Primatology, New York, NY 10024; cDepartment of Anthropology, Lehman College, New York, NY 10468; dDivision of Paleontology, American Museum of Natural History, New York, NY 10024; eSchool of Anthropology, University of Arizona, Tucson, AZ 85721; fInstitute of Ageing and Chronic Disease, University of Liverpool, Liverpool L7 8TX, United Kingdom; gDepartment of Biology, University of Antwerp, 2610 Antwerp, Belgium; hLester E. Fisher Center for the Study and Conservation of Apes, Lincoln Park Zoo, Chicago, IL 60614; iDepartment of Anthropology, Hunter College, New York, NY 10065; and jDepartment of Evolutionary Anthropology, Duke University, Durham, NC 27708 Edited by Carol V. Ward, University of Missouri-Columbia, Columbia, MO, and accepted by Editorial Board Member C. O. Lovejoy March 1, 2018 (received for review September 10, 2017) The evolutionary emergence of humans’ remarkably economical their effects on climbing performance or tested whether these walking gait remains a focus of research and debate, but experi- traits constrain walking and running performance. mentally validated approaches linking locomotor -

Rehabilitation of Soft Tissue Injuries of the Hip and Pelvis T
Donald and Barbara Zucker School of Medicine Journal Articles Academic Works 2014 Rehabilitation of soft tissue injuries of the hip and pelvis T. F. Tyler Northwell Health T. Fukunaga Hofstra Northwell School of Medicine J. Gellert Follow this and additional works at: https://academicworks.medicine.hofstra.edu/articles Recommended Citation Tyler TF, Fukunaga T, Gellert J. Rehabilitation of soft tissue injuries of the hip and pelvis. 2014 Jan 01; 9(6):Article 1396 [ p.]. Available from: https://academicworks.medicine.hofstra.edu/articles/1396. Free full text article. This Article is brought to you for free and open access by Donald and Barbara Zucker School of Medicine Academic Works. It has been accepted for inclusion in Journal Articles by an authorized administrator of Donald and Barbara Zucker School of Medicine Academic Works. INVITED CLINICAL COMMENTARY REHABILITATION OF SOFT TISSUE INJURIES OF THE HIP AND PELVIS Timothy F. Tyler MS, PT, ATC1 Takumi Fukunaga DPT, ATC, CSCS1 Joshua Gellert DPT IJSPT ABSTRACT Soft tissue injuries of the hip and pelvis are common among athletes and can result in significant time loss from sports participation. Rehabilitation of athletes with injuries such as adductor strain, iliopsoas syn- drome, and gluteal tendinopathy starts with identification of known risk factors for injury and comprehen- sive evaluation of the entire kinetic chain. Complex anatomy and overlapping pathologies often make it difficult to determine the primary cause of the pain and dysfunction. The purpose of this clinical commen- tary is to present an impairment-based, stepwise progression in evaluation and treatment of several com- mon soft tissue injuries of the hip and pelvis. -

All About Glutes 1 Table of Contents
All About Glutes 1 Table of Contents Are You Training Your Glutes the Wrong Way? 3 • Anatomy of the Glutes 4 • Functions of the Glutes at the Hip 4 • The Shortcoming of Most Training Programs 5 • Progression and Preventing Knee Valgus 6 • Simple Solution 6 How to Identify and Correct Tight Hip Flexors 8 • What Exactly Are Tight Hip Flexors? 9 • The Hip Flexor Muscle Group 9 • Signs You Have Tight Hip Flexors 10 • What Causes Hip Tightness 10 • Stretches to Loosen up Tight Hip Flexors 10 • Exercises to Strengthen Hip Flexors 11 Pain in the Buttocks When Sitting? Tips to Prevent and Manage 12 Piriformis Syndrome • What is Piriformis Syndrome? 13 • How Does Piriformis Syndrome Happen? 13 • Special Considerations with Clients 14 • Prevention and Pain Management 14 How Do I Build the Perfect Glutes? 16 • Can’t I Just Squat and Lunge? 17 • Your Best Bets to Target the Glutes 18 • Don’t Forget the Legs 18 • Train the Glutes SPECIFICALLY 19 TABLE OF CONTENTS 800.545.4772 WWW.ISSAONLINE.EDU 2 Are You Training Your Glutes the Wrong Way? 800.545.4772 WWW.ISSAONLINE.EDU 3 UNIT ONE These days, the glutes get a lot of attention, and it’s well deserved. When you build and strengthen your glutes in the right way, they not only make your body look better, but they also increase your performance and can diminish knee pain. The problem is most people aren’t taking the best approach to training for the highest level of glute development. Anatomy of the Glutes Let’s start with a little anatomy. -

Effects of a Gluteal Muscles Specific Exercise Program on the Vertical
International Journal of Environmental Research and Public Health Article Effects of a Gluteal Muscles Specific Exercise Program on the Vertical Jump Tomás Gallego-Izquierdo 1, Gerardo Vidal-Aragón 2, Pedro Calderón-Corrales 2, Álvaro Acuña 2, Alexander Achalandabaso-Ochoa 3,* , Agustín Aibar-Almazán 3 , Antonio Martínez-Amat 3 and Daniel Pecos-Martín 1 1 Physiotherapy and Pain Group, Department of Physical Therapy, University of Alcala, 28801 Madrid, Spain; [email protected] (T.G.-I.); [email protected] (D.P.-M.) 2 Physical Therapist, Department of Physical Therapy, University of Alcala, 28801 Madrid, Spain; [email protected] (G.V.-A.); pedrocalderon.fi[email protected] (P.C.-C.); [email protected] (Á.A.) 3 Department of Health Sciences, Faculty of Health Sciences, University of Jaén, 23071 Jaén, Spain.; [email protected] (A.A.-A.); [email protected] (A.M.-A.) * Correspondence: [email protected]; Tel.: +34-953213651 Received: 9 June 2020; Accepted: 23 July 2020; Published: 27 July 2020 Abstract: The vertical jump is a complex movement where many factors are involved in the final result. Currently, how a specific exercise program for gluteal muscles can affect the vertical jump is unknown. So, the aim of this study was to examine the effect of a specific exercise program for the gluteal muscles on a vertical jump. Forty-nine amateur athletes completed an 8-week program. The experimental group received a specific gluteal muscle training program in addition to their regular training routine, whereas the control group received their regular training routine. Jump height, flight time, speed and power were assessed (baseline, postintervention, and 4-week follow-up). -

The Absence of Piriformis Muscle, Combined Muscular Fusion, and Neurovascular Variation in the Gluteal Region
Autopsy Case Report The absence of piriformis muscle, combined muscular fusion, and neurovascular variation in the gluteal region Matheus Coelho Leal1 , João Gabriel Alexander1 , Eduardo Henrique Beber1 , Josemberg da Silva Baptista1 How to cite: Leal MC, Alexander JG, Beber EH, Baptista JS. The absence of piriformis muscle, combined muscular fusion, and neuro-vascular variation in the gluteal region. Autops Case Rep [Internet]. 2021;11:e2020239. https://doi.org/10.4322/ acr.2020.239 ABSTRACT The gluteal region contains important neurovascular and muscular structures with diverse clinical and surgical implications. This paper aims to describe and discuss the clinical importance of a unique variation involving not only the piriformis, gluteus medius, gluteus minimus, obturator internus, and superior gemellus muscles, but also the superior gluteal neurovascular bundle, and sciatic nerve. A routine dissection of a right hemipelvis and its gluteal region of a male cadaver fixed in 10% formalin was performed. During dissection, it was observed a rare presentation of the absence of the piriformis muscle, associated with a tendon fusion between gluteus and obturator internus, and a fusion between gluteus minimus and superior gemellus muscles, along with an unusual topography with the sciatic nerve, which passed through these group of fused muscles. This rare variation stands out with clinical manifestations that are not fully established. Knowing this anatomy is essential to avoid surgical iatrogeny. Keywords Anatomic Variation; Anatomy; Buttocks; Muscle; Piriformis Muscle Syndrome. INTRODUCTION The gluteal region contains important Over the years, these variations have been neurovascular and muscular structures that may classified and distributed into different groups. impose diverse clinical and surgical approaches. -
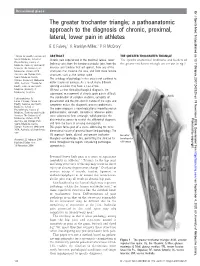
The Greater Trochanter Triangle
Occasional piece Br J Sports Med: first published as 10.1136/bjsm.2007.042325 on 19 November 2008. Downloaded from The greater trochanter triangle; a pathoanatomic approach to the diagnosis of chronic, proximal, lateral, lower pain in athletes E C Falvey,1 A Franklyn-Miller,1 P R McCrory2 1 Centre for Health, Exercise and ABSTRACT THE GREATER TROCHANTER TRIANGLE Sports Medicine, School of Chronic pain experienced in the proximal, lateral, lower The specific anatomical landmarks and borders of Physiotherapy, Faculty of Medicine, Dentistry and Health limb may arise from the femoro-acetabular joint, from the the greater trochanter triangle are set out in fig 1. Sciences The University of muscles and tendons that act upon it, from any of the Melbourne, Victoria 3010 structures that traverse the area, and from more remote Australia and Olympic Park structures such as the lumbar spine. Sports Medicine Centre, The aetiology of pathology in this area is not confined to Olympic Boulevard, Melbourne 3004, Australia; 2 Centre for either trauma or overuse. As a result many different Health, Exercise and Sports sporting activities may have a causal role. Medicine University of Without a clear clinical/pathological diagnosis, the Melbourne, Australia subsequent management of chronic groin pain is difficult. Correspondence to: The combination of complex anatomy, variability of Eanna C Falvey, Centre for presentation and the non-specific nature of the signs and Health, Exercise and Sports symptoms makes the diagnostic process problematic. Medicine, School of Physiotherapy, Faculty of The paper proposes a novel educational model based on Medicine, Dentistry and Health pathoanatomic concepts. -

The Human Gluteus Maximus and Its Role in Running
The Human Gluteus Maximus and its Role in Running The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Lieberman, Daniel E., David A. Raichlen, Herman Pontzer, Dennis M. Bramble, and Elizabeth Cutright-Smith. 2006. The human gluteus maximus and its role in running. Journal of Experimental Biology 209: 2143-2155. Published Version doi:10.1242/jeb.02255 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:3743645 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA 2143 The Journal of Experimental Biology 209, 2143-2155 Published by The Company of Biologists 2006 doi:10.1242/jeb.02255 The human gluteus maximus and its role in running Daniel E. Lieberman1,*, David A. Raichlen1, Herman Pontzer1, Dennis M. Bramble2 and Elizabeth Cutright-Smith3 1Department of Anthropology, Harvard University, 11 Divinity Avenue, Cambridge, MA 02138, USA, 2Department of Biology, University of Utah, Salt Lake City, UT 84112, USA and 3Department of Anthropology, University of Arizona, 1009 E. South Campus Drive PO Box 210030, Tucson, AZ 85721, USA *Author for correspondence (e-mail: [email protected]) Accepted 5 April 2006 Summary The human gluteus maximus is a distinctive muscle in side and to decelerate the swing leg; contractions of the terms of size, anatomy and function compared to apes and stance-side gluteus maximus may also help to control other non-human primates. -

Strengthening Your Hip Muscles Some Exercises May Be Better Than Others
jospt perspectives for patients Strengthening Your Hip Muscles Some Exercises May Be Better Than Others J Orthop Sports Phys Ther 2013;43(2):65. doi:10.2519/jospt.2013.0501 eak hip muscles lead to poor hip motion, and poor difficult to strengthen these muscles without also strengthening hip motion can cause knee, hip, and back pain. By a muscle called the tensor fascia lata, which is located toward exercising to strengthen the hip muscles that con- the front of the hip. Too much activation of that muscle may Wtrol how your hip moves, you can reduce your pain create unwanted hip motion that may worsen knee, hip, or back in these parts of your body. The 2 key muscles to include in your pain. A study published in the February 2013 issue of JOSPT exercise program are the gluteus maximus (the chief muscle on provides information intended to help physical therapists and the back of your hip—your buttocks) and the gluteus medius their patients select exercises that target the buttock muscles (the main muscle on the side of your hip). However, it is often without causing other unwanted muscle actions. NEW INSIGHTS In this study, the researchers had 20 healthy people perform 11 different hip exercises commonly used for both fitness and rehabilitation. While the participants performed the exercises, fine wires were used to record the amount of electrical activity within the 3 muscles. This indicated how much each muscle was working. The researchers’ goal was to discover which exercises used the gluteus maximus and gluteus medius muscles the most, while minimizing the action of the tensor fascia lata. -

Pelvis & Thigh
Pelvis & Thigh 6 After meeting a stranger, you soon begin to palpate their piriformis Topographical Views 276 muscle (located deep in the posterior buttock). You certainly wouldn’t try Exploring the Skin and Fascia 277 this in “everyday life,” but in patient care settings this level of familiarity is Bones of the Pelvis and Thigh 278 commonplace—and welcomed by a client with a hypercontracted piriformis. Bony Landmarks of the Pelvis Touch is a unique privilege afforded to health care providers. As such, we and Thigh 279 need to be mindful of the trust our clients have in us. One way to insure this Overview: Bony Landmark Trails 284 is through good communication skills. For instance, working the adductors Overview: Muscles of the and gluteal region requires a practitioner to provide ample explanation as to Pelvis and Thigh 296 the rationale, need, and goals of working these intimate areas of the body. Synergists—Muscles Working This chapter might pose new challenges for you, as we will be palpating Together 302 structures close to intimate areas. Muscles of the Pelvis and Thigh 306 Ligaments and Other Before proceeding, consider the following questions: Structures of the Pelvis and Thigh 336 E Have you ever been anxious to undergo a physical exam? Was there anything the practitioner did or could have done to alleviate this anxiety? Consider multiple elements, including both verbal and nonverbal communication, draping, physical pressure, and pace. E Tissues and landmarks found in the pelvis and thigh tend to be significantly larger than those discussed in previous chapters. How might your palpation techniques need to change? E Also, how might you properly and comfortably position your patient to access structures needing to be palpated. -

Gluteal Region-I
Gluteal Region-I Dr Garima Sehgal Associate Professor King George’s Medical University UP, Lucknow Intramuscular (IM) gluteal injections are a commonly used method of administering medication within clinical medicine. Learning Objectives By the end of this teaching session on Gluteal region – I all the MBBS 1st year students must be able to: • Enumerate the boundaries of gluteal region • Enumerate the foramina of gluteal region • Describe the cutaneous innervation of gluteal region • Enumerate the structures in the gluteal region (bones, ligaments, muscles, vessels , nerves) • Describe the origin, insertion, nerve supply & actions of gluteal muscles • Name the key muscle of gluteal region • Describe the origin, insertion, nerve supply & actions of short muscles of the gluteal region • Discuss applied anatomy of muscles of gluteal region Gluteal region BOUNDARIES: Superior: Iliac crest S3 Inferior : Gluteal fold (lower limit of rounded buttock) Lateral : Line joining ASIS to front of greater trochanter Medial : natal cleft between buttocks Structures in the Gluteal region • Bones & joints • Ligaments Thickest muscle- • Muscles Gluteus maximus • Vessels • Nerves Thickest nerve Sciatic nerve • Bursae Bones & Joints of the gluteal region • Dorsal surface of sacrum • Coccyx • Gluteal surface of Ilium • Ischium (ischial tuberosity) • Upper end of femur • Posterior aspect of hip joint • Sacrococcygeal & sacroiliac joint Skeletal background features- Gluteal lines on hip bone Skeletal background features- Features on posterior surface of upper -

Outcomes of Non-Operative Management of Deep Gluteal
Schröder et al. J Musculoskelet Disord Treat 2016, 2:012 Journal of Volume 2 | Issue 2 Musculoskeletal Disorders and Treatment Case Series: Open Access Outcomes of Non-operative Management of Deep Gluteal Syndrome - A Case Series of Six Patients Ricardo Gonçalves Schröder1*, Rob Roy L Martin2, Valerie L Bobb3, Anthony Nicholas Khoury1,4, Ian James Palmer1 and Hal David Martin1 1Hip Preservation Center, Baylor Scott & White Health, Dallas, TX, USA 2Department of Physical Therapy, Duquesne University, Pittsburgh, PA, USA 3Baylor Institute for Rehabilitation - OP, Baylor Scott & White Health, Dallas, TX, USA 4Bioengineering Department, University of Texas at Arlington, Arlington, TX, USA *Corresponding author: Ricardo Gonçalves Schröder, Hip Preservation Center, Baylor Scott & White Health, 3900 Junius St. Suite 705, Dallas, Texas - 75246, USA, E-mail: [email protected] musculoskeletal, psychological and social factors. A multiprofissional Abstract approach may be necessary to manage DGS addressing these factors. Introduction: An increased understanding of posterior hip anatomy and biomechanics has led to advances in the diagnosis DGS is a condition which patients frequently present with and management of Deep Gluteal Syndrome (DGS). Currently, posterior hip pain associated to inability to sit for 30 minutes, radicular there is limited information detailing the successful conservative pain of the lower back or hip, and paresthesia’s of the affected leg. management of DGS. The feeling of motor weakness can also present during standing Material & methods: The detailed history, physical examination, activities [2,3,8,9]. Due to the anatomical variability surrounding imaging, diagnostic testing, and physical therapy of six subjects the sciatic nerve, the etiology of the DGS can result from multiple diagnosed with deep gluteal syndrome were retrospectively structures such as bone, fibrous scar bands, and muscles [10-12]. -

Recommendation of the Best Site Based on the Distribution Pattern of Extramuscular and Intramusular Nerves for Gluteal Muscle Injection
Int. J. Morphol., 38(4):975-982, 2020. Recommendation of the Best Site Based on the Distribution Pattern of Extramuscular and Intramusular Nerves for Gluteal Muscle Injection Recomendación del Mejor Sitio para la Inyección del Músculo Glúteo Basado en el Patrón de Distribución de los Nervios Extramusculares e Intramusculares Junxi Wu1; Yanzhen Cai1; Ai Cao1; Yu Bi1; Xiangnan Hu2 & Shengbo Yang2 WU, J.; CAI, Y.; CAO, A.; BI, Y.; HU, X. & YANG, S. Recommendation of the best site based on the distribution pattern of extramuscular and intramuscular nerves for gluteal muscle injection. Int. J. Morphol., 38(4):975-982, 2020. SUMMARY: To reveal the extra- and intramuscular nerve distribution patterns of the gluteus maximus, medius, and minimus, and to provide guidance for gluteal muscle injection in order to avoid nerve injury. Ten adult and 10 child cadavers were used. The superior and inferior gluteal nerves innervating the gluteus maximus, medius, and minimus were dissected, exposed, and sutured in-situ on the muscle. The three gluteal muscles were removed, and the distribution patterns of the intramuscular nerves were revealed by modified Sihler’s nerve staining. The nerve distribution pattern was returned to the corresponding position in the body, and the patterns in the four quadrants of the buttock were analyzed. There were 3–12 extramuscular nerve branches of the gluteus maximus, medius, and minimus. After entering the muscle, these nerve branches arborized and anastomosed to form an arc-shaped, nerve-dense zone. The nerve distribution was most dense in the inferomedial region of the superolateral quadrant and the inferolateral region of the superomedial quadrant of the buttocks.