Partial Order Ranking of Organophosphates with Special
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

US EPA Environmental Technology Verification Statement Removal of Chemical Contaminants in Drinking Water Watts Premier Inc
THE ENVIRONMENTAL TECHNOLOGY VERIFICATION PROGRAM U.S. Environmental Protection Agency NSF International ETV Joint Verification Statement TECHNOLOGY TYPE: POINT-OF-USE DRINKING WATER TREATMENT SYSTEM APPLICATION: REMOVAL OF CHEMICAL CONTAMINANTS IN DRINKING WATER PRODUCT NAME: WATTS PREMIER WP-4V COMPANY: WATTS PREMIER, INC. ADDRESS: 1725 WEST WILLIAMS DR. SUITE C-20 PHOENIX, AZ 85027 PHONE: 800-752-5582 NSF International (NSF) manages the Drinking Water Systems (DWS) Center under the U.S. Environmental Protection Agency’s (EPA) Environmental Technology Verification (ETV) Program. The DWS Center recently evaluated the performance of the Watts Premier WP-4V point-of-use (POU) drinking water treatment system. NSF performed all of the testing activities, and also authored the verification report and this verification statement. The verification report contains a comprehensive description of the test. EPA created the ETV Program to facilitate the deployment of innovative or improved environmental technologies through performance verification and dissemination of information. The goal of the ETV Program is to further environmental protection by accelerating the acceptance and use of improved and more cost-effective technologies. ETV seeks to achieve this goal by providing high-quality, peer- reviewed data on technology performance to those involved in the design, distribution, permitting, purchase, and use of environmental technologies. ETV works in partnership with recognized standards and testing organizations, stakeholder groups (consisting of buyers, vendor organizations, and permitters), and with the full participation of individual technology developers. The program evaluates the performance of innovative technologies by developing test plans that are responsive to the needs of stakeholders, conducting field or laboratory tests (as appropriate), collecting and analyzing data, and preparing peer reviewed reports. -

"U/^ ((*.U \ BIBLIOTHEEK STARINGGEBOUW Volatilization of Tri-Allate, Ethoprophos and Parathion Measured with Four Methods After Spraying on a Sandy Soil
"u/^ ((*.u \ BIBLIOTHEEK STARINGGEBOUW Volatilization of tri-allate, ethoprophos and parathion measured with four methods after spraying on a sandy soil G. Bor F. van den Berg J.H. Smelt R.A. Smidt A.E. van de Peppel-Groen M. Leistra Report 104 DLO Winand Staring Centre, Wageningen (The Netherlands), 1995 1 h FEB. 1998 0000' ABSTRACT Bor, G., F. van den Berg, J.H. Smelt, R.A. Smidt, A.E. van de Peppel-Groen, M. Leistra, 1995. Volatilization of tri-allate, ethoprophos and parathion measured withfour methods after spraying on a sandy soil. Wageningen (The Netherlands), DLO Winand Staring Centre. Report 104. 62 pp.; 9 Figs; 6 Tables; 14 Refs; 3 Annex. At about eleven times after application of tri-allate, ethoprophos and parathion to a sandy soil, their rates of volatilization were determined with the aerodynamic method (AD),th e Bowen-ratio method (BR), the theoretical-profile method (TP) and the Box method (B). The volatilization was highest for tri-allate and lowest for parathion. On the first day after application, the volatilization rate decreased sharply,bu tthereafte r the decreasewa smor egradual .Th edifference s involatilizatio n rate asdetermine d withth eAD ,B R andT Pmethod s werecomparativel y small.Th erate sdetermine d with the Box method were mostly lower than those determined with the other methods. Keywords: aerodynamic method, air quality, air sampling, Bowen ratio method, Box method, field experiment, gas chromatography, pesticide, polystyrene, theoretical-profile method, XAD ISSN 0927-4537 ©1995 DLO Winand Staring Centre for Integrated Land, Soil and Water Research (SC-DLO) P.O. -

Monocrotophos Proposed AEGL Document
MONOCROTOPHOS Page 1 of 32 Proposed 10/2009 v.1 1 2 3 4 ACUTE EXPOSURE GUIDELINE LEVELS 5 (AEGLs) 6 7 8 PROPOSED 9 10 11 12 13 MONOCROTOPHOS 14 (CAS Reg. No. 6923-22-4) 15 16 17 18 19 20 MONOCROTOPHOS Page 2 of 32 Proposed 10/2009 v.1 1 2 PREFACE 3 4 Under the authority of the Federal Advisory Committee Act (FACA) P. L. 92-463 of 5 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous 6 Substances (NAC/AEGL Committee) has been established to identify, review and interpret 7 relevant toxicologic and other scientific data and develop AEGLs for high priority, acutely toxic 8 chemicals. 9 10 AEGLs represent threshold exposure limits for the general public and are applicable to 11 emergency exposure periods ranging from 10 minutes to 8 hours. Three levels C AEGL-1, 12 AEGL-2 and AEGL-3 C are developed for each of five exposure periods (10 and 30 minutes, 1 13 hour, 4 hours, and 8 hours) and are distinguished by varying degree of severity of toxic effects. 14 The three AEGLs are defined as follows: 15 16 AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per 17 cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general 18 population, including susceptible individuals, could experience notable discomfort, irritation, or 19 certain asymptomatic, non-sensory effects. However, the effects are not disabling and are 20 transient and reversible upon cessation of exposure. -

Downloads/Pgm Hydrus1d/HYDRUS-4.16.Pdf (Accessed on 20 April 2019)
water Article Assessment of the Environmental Risk of Pesticides Leaching at the Watershed Scale under Arid Climatic Conditions and Low Recharge Rates Hesham M. Ibrahim 1,2,* and Ali M. Al-Turki 1 1 Department of Soil Science, College of Food and Agricultural Sciences, King Saud University, P.O. Box 2460, Riyadh 11451, Saudi Arabia; [email protected] 2 Department of Soils and Water, Faculty of Agriculture, Suez Canal University, Ismailia 41522, Egypt * Correspondence: [email protected]; Tel.: +966-501728656 Received: 5 January 2020; Accepted: 2 February 2020; Published: 5 February 2020 Abstract: The assessment of the vulnerability of soil and groundwater resources to pesticide contamination is important to reduce the risk of environmental pollution. The applicability of the expanded attenuation factor (EAF) to assess leaching potential of 30 pesticides was investigated under 1 four recharge rates (0.0003–0.002 m d− ) in the arid environment of the Jazan watershed. EAF results revealed that Picloram, Carbofuran, Monocrotophos, and 2,4-D pesticides showed high leaching potential, mainly because of their low KOC, and relatively longer t1/2. In addition, medium leaching potential was observed with six more pesticides (Atrazine, Aldicarb, Simazine, Methomyl, Oxamyl, and Lindane). Regardless of the recharge rate, all other pesticides showed a very low leaching potential in the Jazan watershed. Sensitivity analysis revealed that the output of the EAF index is most sensitive to the fraction of organic carbon ( foc), water content at field capacity (θFC ), recharge rate (q), and partition coefficient (KOC), and least sensitive to soil bulk density (ρb) and air-filled porosity (na). -

Toxic Influence of Diazinon As an Organophosphate Pesticide on Parameters of Dry Matter Degradability According to in Situ Technique M
International Journal of Basic & Applied Sciences IJBAS-IJENS Vol:12 No:06 229 Toxic Influence of Diazinon as an Organophosphate Pesticide on Parameters of Dry Matter Degradability According to in Situ Technique M. Kazemi*, A. M. Tahmasbi, R. Valizadeh, A. A. Naserian Department of Animal Science, Excellence Center for Animal Science, Faculty of Agriculture, Ferdowsi University of Mashhad, P. O. Box 91775-1163, Mashhad, Islamic Republic of Iran. *Corresponding author email: [email protected] Abstract-- Many feed and forages are exposed to low, medium metabolizing phosphate insecticides in vitro. Parathion was or high levels of organophosphorous (OP) pesticides. It is unclear reduced to aminoparathion by bovine rumen fluid. Kutches et whether these exposures (for example diazinon as an OP al. [15] reported that toxaphene was the most inhibitory of the pesticide) impact dry matter degradability parameters. So, pesticides tested and resulted in greater decreases of the in different levels (0, 0.7, 2.8 and 5.6 mg) of diazinon as an OP vitro dry matter disappearance, volatile fatty acids and rumen pesticide with different levels of calcium bentonite (CB) (0 and 100 mg) as a toxin binder were tested for their toxicological protozoa. Inhibitory effects of OP pesticides such as effects on in vitro dry matter disappearance. Also we investigated phosphomidon, chlorpyrifos and phosalone on tetrahymena whether CB can inactivate the probably deleterious effects of Spp. Were reported by Hoskin et al. [10]. Dauterman et al. [7] contaminated forage with diazinon in the situ procedure or not. treated rats and cattle with radioactive dimethoate and The study indicated that effect of diazinon with adding the analyzed blood, tissue, milk, and excreta. -

Agents for Defense Against Chemical Warfare: Reactivators of Acetylcholinesterase Inhibited with Neurotoxic Organophosphorus Compounds **
Mil. Med. Sci. Lett. (Voj. Zdrav. Listy) 2015, vol. 84(3), p. 115-127 ISSN 0372-7025 DOI: 10.31482/mmsl.2015.013 REVIEW ARTICLE AGENTS FOR DEFENSE AGAINST CHEMICAL WARFARE: REACTIVATORS OF ACETYLCHOLINESTERASE INHIBITED WITH NEUROTOXIC ORGANOPHOSPHORUS COMPOUNDS ** Petronilho, E. C., Figueroa-Villar, J. D. Chemistry Engineering Section, Medicinal Chemistry Group, Military Institute of Engineering, Praça General Tibúrcio, 80, Urca, 22290-270, Rio de Janeiro, RJ, Brazil. Received 30 th April 2015. Revised 7 th July 2015. Published 4 th September 2015. Summary The chemical warfare agents and neurotoxic agents are an important threat to people all over the world, and require special attention because they are highly dangerous. Most of these agents are neurotoxic organophosphorus compounds (OP), which inhibit the enzyme acetylcholinesterase (AChE), which is responsible for controlling the transmission of nerve impulses. To be inhibited by these compounds, AChE can sometimes be reactivated using cationic oximes, which are the most used substances for this reactivation. Until today there have not been discovered agents for complete treatment of poisoning by all OPs. For this reason, the treatment of intoxicated people requires the determination of the absorbed OP, in order to select the appropriate activator, a process that usually requires long time and may cause death. Therefore, this study aims to do a review on the OPs used as chemical warfare agents and the process of inhibition and reactivation of AChE, especially to motivate the development of new agents for defense against chemical weapons, a process that is very important for protecting all humanity. Key words: acetylcholinesterase; AChE reactivators; organophosphorus; oximes; warfare agents INTRODUCTION pounds (OP), which are highly toxic, allowing their use with small quantities in order to cause seizures The use of chemical warfare agents is a major and death. -

EPA Method 538: Determination of Selected Organic Contaminants in Drinking Water with Direct Aqueous Injection LC/MS/MS
EPA Method 538: Determination of Selected Organic Contaminants in Drinking Water with Direct Aqueous Injection LC/MS/MS E. Michael Thurman and Imma Ferrer Center for Environmental Mass Spectrometry University of Colorado Boulder, CO, USA Confidentiality Label 1 March 20, 2012 Abstract EPA Method 538 is a new method from EPA for organophosphate pesticides in drinking water. It uses direct aqueous injection; thus, no sample preparation is needed. We use both UHPLC (Agilent 1290) and MS/MS (Agilent 6460) analysis for rapid analysis and sensitive detection with ng/L limits of detection. A second MRM is added for more reliable identification. Confidentiality Label 2 March 20, 2012 Hypothesis Direct injection of organophosphate pesticides (EPA Method 538) will work by UHPLC (Agilent Model 1290) and LC/MS/MS with Jetstream (Agilent Model 6460) with trace level detection at ng/L concentrations. Confidentiality Label 3 March 20, 2012 1. Introduction-Summary 1.1 EPA Method 538 (published in November 2009 by Shoemaker) deals with Organophosphate pesticides in drinking water (1) and one other contaminant, quinoline. 1.2 The method consists of 10 compounds: acephate, aldicarb, aldicarb sulfoxide, dicrotophos, diisopropylmethylphosphonate (DIMP), fenamiphos sulfone, fenamiphos sulfoxide, methamidophos, oxydemeton methyl, quinoline, and thiofanox with 5 labeled internal standards. 1.3 Direct aqueous injection is used with a large volume sample of 100 microliters; thus, no sample preparation is needed. 1.4 Because solid phase extraction (i.e. concentration of the sample is not carried out) suppression is mimimized in the analysis. 1.5 Part-per-Trillion Detection Limits. Confidentiality Label 4 March 20, 2012 Introduction 1.1: EPA Method 538: Determination of Selected Organic Contaminants in Drinking Water by Direct Aqueous Injection by Jody Shoemaker, EPA Cincinnati, OH [email protected] 513-569-7298 Confidentiality Label 5 March 20, 2012 Introduction: 1.2. -

Phytotoxicity of Some Organophosphate Insecticides to Oughly with Air Dry, Sieved Soil, to Pro Duce Rates of 37.5, 75 and ISO Mg Aj
Plant Protection Quarterly VoI.7(1} 1992 23 -------------------------------- ties of each pesticide were shaken thor Phytotoxicity of some organophosphate insecticides to oughly with air dry, sieved soil, to pro duce rates of 37.5, 75 and ISO mg aj. L-' onions and carrots during germination and emergence soil. Each pot constituted one plot. If it is assumed that in a field situation, P.J. Sinclair, New South Wales Agriculture, Horticultural Research and band-in-furrow treatment would treat a Advisory Station, Griffith, New South Wales 2680, Australia. strip 50 mm wide by 20 mm deep, the rates tested would correspond to 37.5, 75 R.J. Neeson and P.A. Williams, New South Wales Agriculture, Agricultural and 150 mg a.i. m" row, or 0.5,1.0 and 2.0 institute, Yanco, New South Wales 2703, Australia. kg a.i. ha" at a row spacing of 75 cm. The low and medium rates are then compara Summary ble to ra tes used in the field by Getzin The phytotoxicity of some commonly phytotoxicity from carbofuran applied to (1973), Thompson et al. (1981) and used insecticides to onions (Allium cepa) onions as a seed dressing. Chlorpyrifos is Goodyer et al. (1989) . and carrots (Daucus carota) during es generally non-phytotoxic at recom Daily counts of emerged seedlings were tablishment was assessed in pot trials. mended rates and methods of application, made at 7 to 18 days and at 21 days after Terbufos, ethoprophos, phoxirn and but some crops are especially sensitive to first watering. These data were used to de carbofuran (all 10% a.L granular fonnu it during the seedling stage or if the termine the total number of seedlings lations) and chlorpyrifos (25% a.i. -

Development and Validation of a Method for the Simultaneous
CORE Metadata, citation and similar papers at core.ac.uk Provided by UGD Academic Repository American Journal of Applie d Chemistry 2014; 2(4): 46-54 Published online August 10, 2014 (http://www.sciencepublishinggroup.com/j/ajac) doi: 10.11648/j.ajac.20140204.11 ISSN: 2330-8753 (Print); ISSN: 2330-8745 (Online) Development and validation of a method for the simultaneous determination of 20 organophosphorus pesticide residues in corn by accelerated solvent extraction and gas chromatography with nitrogen phosphorus detection Vesna Kostik *, Biljana Gjorgeska, Bistra Angelovska Medical Faculty, Department of Pharmacy, University “Goce Delchev”, Shtip, Republic of Macedonia Email address: [email protected] (V. Kostik), [email protected] (B. Gjorgeska), [email protected] (B. Angelovska) To cite this article: Vesna Kostik, Biljana Gjorgeska, Bistra Angelovska. Development and Validation of a Method for the Simultaneous Determination of 20 Organophosphorus Pesticide Residues in Corn by Accelerated Solvent Extraction and Gas Chromatography with Nitrogen Phosphorus Detection. American Journal of Applied Chemistry. Vol. 2, No. 4, 2014, pp. 46-54. doi: 10.11648/j.ajac.20140204.11 Abstract: The method for simultaneous determination of 20 organophosphorus pesticide residues in corn samples has been developed and validated. For the extraction of organophosporus pesticide residues from the samples, the accelerated solvent technique with the mixture of dichloromethane: acetone (1:1, V/V ) was used. Clean up was done using liquid – liquid extraction with n – hexane, followed by solid phase extraction on primary secondary amine adsorbent, and elution with the mixture of acetone: toluene (65:35). The determination of the pesticides was carried out by gas chromatography with nitrogen phosphorus detection. -

The Effect of Processing on 14 C-Chlofenvinphos Residues In
EG0800342 The Effect of Processing on 14C- Chlorfenvinphos Residues in Maize Oil and Bioavailability of its Cake Residues on Rats F. Mahdy, S. El-Maghraby National Research Centre, Dokki, Giza, Egypt ABSTRACT Maize seed obtained from 14C-chlorfenvinphos treated plants contained 0.12 % of the applied dose. The insecticide residues in crude oil, methanol and coke amounted to 10 %, 6 % and 69 %, respectively of original residues inside the seeds.The 14C-activity in the crude oil could be a gradual reduced by the refining processes. The alkali treatment and bleaching steps are more effective steps in the refining processes remove about (63 %). The refined oil contained only about 17 % of the 14C-residues originally present. The major residues in processed oil contain parent compound, in addition to five metabolites of the insecticide. When rats fed the extracted seeds (cake), the bound residues were found to be considerably bioavailable. After feeding rats for 5 days with the cake, a substantial amount of 14C-residues was eliminated in the urine (59.5 %), while about 20 % was excreted in the feces. About 15 % of the radioactivity was distribution among various organs. Keywords: 14C-chlorfenvinphos, maize oil, refining processes, residues, bioavailability. INTRODUCTION Pesticides are of interest as residues because of their widespread use in a variety of crops for field and post-harvest protection and because with their degradation products, they could be a potential health hazard. Past investigations suggest that most organochlorine and organophosphorus pesticide in edible oils can be reduced considerably by a chemical refining process, but pyrethroid pesticides remain to a certain extent (1-6). -

Determination of Organophosphorus Pesticide Residues in Vegetables Using Solid Phase Micro-Extraction Coupled with Gas Chromatography–flame Photometric Detector
Arabian Journal of Chemistry (2015) xxx, xxx–xxx King Saud University Arabian Journal of Chemistry www.ksu.edu.sa www.sciencedirect.com ORIGINAL ARTICLE Determination of organophosphorus pesticide residues in vegetables using solid phase micro-extraction coupled with gas chromatography–flame photometric detector Haizarul Aida Sapahin, Ahmad Makahleh *, Bahruddin Saad * School of Chemical Sciences, Universiti Sains Malaysia, 11800 Minden, Penang, Malaysia Received 17 July 2014; accepted 9 December 2014 KEYWORDS Abstract An adequate and simple analytical method based on solid-phase microextraction Organophosphorus pesticide; (SPME) followed by gas chromatography–flame photometric detection (GC–FPD) for the determi- Direct immersed-solid phase nation of eleven organophosphorus pesticide residues (i.e., ethoprophos, sulfotep, diazinon, tolclo- microextraction; fos-methyl, fenitrothion, chlorpyrifos, isofenphos, methidathion, ethion, triazophos, leptophos) in Gas chromatography–flame vegetables samples (cabbage, kale and mustard) was developed. Important parameters that influ- photometric detector; ence the extraction efficiency (i.e., fibre type, extraction modes, extraction time, salt addition, Vegetables desorption time and temperature) were systematically investigated. Four types of commercially available fibres (i.e., 50/30 lm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS), 65 lm polydimethylsiloxane/divinylbenzene (PDMS/DVB), 100 lm polydimethylsiloxane (PDMS), and 85 lm polyacrylate (PA)) were evaluated. PA fibre exhibited the best performance and was used for the rest of the studies. The optimised extraction conditions were: extraction time, 30 min at room temperature; stirring speed, 1275 rpm; salt content, 10% NaCl; desorption time and temper- ature, 11 min at 260 °C; and no pH adjustment of the sample extract. The method was validated over the range 0.1–100 lg/L. -
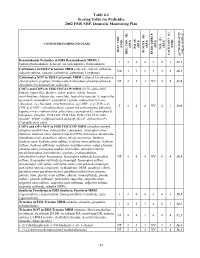
2002 NRP Section 6, Tables 6.1 Through
Table 6.1 Scoring Table for Pesticides 2002 FSIS NRP, Domestic Monitoring Plan } +1 0.05] COMPOUND/COMPOUND CLASS * ) (EPA) (EPA) (EPA) (EPA) (EPA) (FSIS) (FSIS) PSI (P) TOX.(T) L-1 HIST. VIOL. BIOCON. (B) {[( (2*R+P+B)/4]*T} REG. CON. (R) * ENDO. DISRUP. LACK INFO. (L) LACK INFO. {[ Benzimidazole Pesticides in FSIS Benzimidazole MRM (5- 131434312.1 hydroxythiabendazole, benomyl (as carbendazim), thiabendazole) Carbamates in FSIS Carbamate MRM (aldicarb, aldicarb sulfoxide, NA44234416.1 aldicarb sulfone, carbaryl, carbofuran, carbofuran 3-hydroxy) Carbamates NOT in FSIS Carbamate MRM (carbaryl 5,6-dihydroxy, chlorpropham, propham, thiobencarb, 4-chlorobenzylmethylsulfone,4- NT 4 1 3 NV 4 4 13.8 chlorobenzylmethylsulfone sulfoxide) CHC's and COP's in FSIS CHC/COP MRM (HCB, alpha-BHC, lindane, heptachlor, dieldrin, aldrin, endrin, ronnel, linuron, oxychlordane, chlorpyrifos, nonachlor, heptachlor epoxide A, heptachlor epoxide B, endosulfan I, endosulfan I sulfate, endosulfan II, trans- chlordane, cis-chlordane, chlorfenvinphos, p,p'-DDE, p, p'-TDE, o,p'- 3444NV4116.0 DDT, p,p'-DDT, carbophenothion, captan, tetrachlorvinphos [stirofos], kepone, mirex, methoxychlor, phosalone, coumaphos-O, coumaphos-S, toxaphene, famphur, PCB 1242, PCB 1248, PCB 1254, PCB 1260, dicofol*, PBBs*, polybrominated diphenyl ethers*, deltamethrin*) (*identification only) COP's and OP's NOT in FSIS CHC/COP MRM (azinphos-methyl, azinphos-methyl oxon, chlorpyrifos, coumaphos, coumaphos oxon, diazinon, diazinon oxon, diazinon met G-27550, dichlorvos, dimethoate, dimethoate