Combinatorial Amygdalar Inputs to Hippocampal Domains and Q Hypothalamic Behavior Systems Gorica D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparative Analysis of the Distribution of Immunoreactive Orexin a and B in the Brains of Nocturnal and Diurnal Rodents Joshua P Nixon*1,2 and Laura Smale1
Behavioral and Brain Functions BioMed Central Research Open Access A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents Joshua P Nixon*1,2 and Laura Smale1 Address: 1Department of Zoology, Michigan State University, 203 Natural Science Building, East Lansing, MI 48824-1115 USA and 2Department of Food Science and Nutrition and Minnesota Craniofacial Research Training Program (MinnCResT), 17-164 Moos Tower, 515 Delaware St. SE, Minneapolis, MN 55455-0357 USA Email: Joshua P Nixon* - [email protected]; Laura Smale - [email protected] * Corresponding author Published: 13 June 2007 Received: 19 November 2006 Accepted: 13 June 2007 Behavioral and Brain Functions 2007, 3:28 doi:10.1186/1744-9081-3-28 This article is available from: http://www.behavioralandbrainfunctions.com/content/3/1/28 © 2007 Nixon and Smale; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: The orexins (hypocretins) are a family of peptides found primarily in neurons in the lateral hypothalamus. Although the orexinergic system is generally thought to be the same across species, the orexins are involved in behaviors which show considerable interspecific variability. There are few direct cross-species comparisons of the distributions of cells and fibers containing these peptides. Here, we addressed the possibility that there might be important species differences by systematically examining and directly comparing the distribution of orexinergic neurons and fibers within the forebrains of species with very different patterns of sleep-wake behavior. -

Diencephalon Diencephalon
Diencephalon Diencephalon • Thalamus dorsal thalamus • Hypothalamus pituitary gland • Epithalamus habenular nucleus and commissure pineal gland • Subthalamus ventral thalamus subthalamic nucleus (STN) field of Forel Diencephalon dorsal surface Diencephalon ventral surface Diencephalon Medial Surface THALAMUS Function of the Thalamus • Sensory relay – ALL sensory information (except smell) • Motor integration – Input from cortex, cerebellum and basal ganglia • Arousal – Part of reticular activating system • Pain modulation – All nociceptive information • Memory & behavior – Lesions are disruptive Classification of Thalamic Nuclei I. Lateral Nuclear Group II. Medial Nuclear Group III. Anterior Nuclear Group IV. Posterior Nuclear Group V. Metathalamic Nuclear Group VI. Intralaminar Nuclear Group VII. Thalamic Reticular Nucleus Classification of Thalamic Nuclei LATERAL NUCLEAR GROUP Ventral Nuclear Group Ventral Posterior Nucleus (VP) ventral posterolateral nucleus (VPL) ventral posteromedial nucleus (VPM) Input to the Thalamus Sensory relay - Ventral posterior group all sensation from body and head, including pain Projections from the Thalamus Sensory relay Ventral posterior group all sensation from body and head, including pain LATERAL NUCLEAR GROUP Ventral Lateral Nucleus Ventral Anterior Nucleus Input to the Thalamus Motor control and integration Projections from the Thalamus Motor control and integration LATERAL NUCLEAR GROUP Prefrontal SMA MI, PM SI Ventral Nuclear Group SNr TTT GPi Cbll ML, STT Lateral Dorsal Nuclear Group Lateral -

Projections of the Paraventricular and Paratenial Nuclei of the Dorsal Midline Thalamus in the Rat
THE JOURNAL OF COMPARATIVE NEUROLOGY 508:212–237 (2008) Projections of the Paraventricular and Paratenial Nuclei of the Dorsal Midline Thalamus in the Rat ROBERT P. VERTES* AND WALTER B. HOOVER Center for Complex Systems and Brain Sciences, Florida Atlantic University, Boca Raton, Florida 33431 ABSTRACT The paraventricular (PV) and paratenial (PT) nuclei are prominent cell groups of the midline thalamus. To our knowledge, only a single early report has examined PV projections and no previous study has comprehensively analyzed PT projections. By using the antero- grade anatomical tracer, Phaseolus vulgaris leucoagglutinin, and the retrograde tracer, FluoroGold, we examined the efferent projections of PV and PT. We showed that the output of PV is virtually directed to a discrete set of limbic forebrain structures, including ‘limbic’ regions of the cortex. These include the infralimbic, prelimbic, dorsal agranular insular, and entorhinal cortices, the ventral subiculum of the hippocampus, dorsal tenia tecta, claustrum, lateral septum, dorsal striatum, nucleus accumbens (core and shell), olfactory tubercle, bed nucleus of stria terminalis (BST), medial, central, cortical, and basal nuclei of amygdala, and the suprachiasmatic, arcuate, and dorsomedial nuclei of the hypothalamus. The posterior PV distributes more heavily than the anterior PV to the dorsal striatum and to the central and basal nuclei of amygdala. PT projections significantly overlap with those of PV, with some important differences. PT distributes less heavily than PV to BST and to the amygdala, but much more densely to the medial prefrontal and entorhinal cortices and to the ventral subiculum of hippocampus. As described herein, PV/PT receive a vast array of afferents from the brainstem, hypothalamus, and limbic forebrain, related to arousal and attentive states of the animal, and would appear to channel that information to structures of the limbic forebrain in the selection of appropriate responses to changing environmental conditions. -

Neural Projections from Nucleus Accumbens To
0270.6474/83/0301-0189$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 3, No. 1, pp. 189-202 Printed in U.S.A. January 1983 NEURAL PROJECTIONS FROM NUCLEUS ACCUMBENS TO GLOBUS PALLIDUS, SUBSTANTIA INNOMINATA, AND LATERAL PREOPTIC- LATERAL HYPOTHALAMIC AREA: AN ANATOMICAL AND ELECTROPHYSIOLOGICAL INVESTIGATION IN THE RAT’ G. J. MOGENSON, L. W. SWANSON,’ AND M. WU Department of Physiology, University of Western Ontario, London, Ontario, Canada N6A 5Cl and The Salk Institute, San Diego, California 92138 Received April 12, 1982; Revised July 21, 1982; Accepted July 26, 1982 Abstract The anatomical organization and electrophysiological characteristics of a projection from the nucleus accumbens to anteroventral parts of the globus pallidus and to a subpallidal region that includes the substantia innominata (SI), the lateral preoptic area (LPO), and anterior parts of the lateral hypothalamic area (LHA) were investigated in the rat. Autoradiographic experiments, with injections of 3H-proline into different sites in the nucleus accumbens and adjacent caudoputamen, indicate that the descending fibers are organized topographically along both mediolateral and dorsoventral gradients, although labeled fibers from adjacent regions of the nucleus accumbens overlap considerably in the ventral globus pallidus and subpallidal region. Injections confined to the caudoputaman only labeled fibers in the globus pallidus. Retrograde transport experiments with the marker true blue confirmed that only the nucleus accumbens projects to the subpallidal region and that the caudoputamen projects upon the glubus pallidus in a topographically organized manner. In electrophysiological recording experiments single pulse stimulation (0.1 to 0.7 mA; 0.15 msec duration) of the nucleus accumbens changed the discharge rate of single neurons in the ventral globus pallidus and in the SI, LPO, and LHA. -

White Matter Anatomy: What the Radiologist Needs to Know
White Matter Anatomy What the Radiologist Needs to Know Victor Wycoco, MBBS, FRANZCRa, Manohar Shroff, MD, DABR, FRCPCa,*, Sniya Sudhakar, MBBS, DNB, MDb, Wayne Lee, MSca KEYWORDS Diffusion tensor imaging (DTI) White matter tracts Projection fibers Association Fibers Commissural fibers KEY POINTS Diffusion tensor imaging (DTI) has emerged as an excellent tool for in vivo demonstration of white matter microstructure and has revolutionized our understanding of the same. Information on normal connectivity and relations of different white matter networks and their role in different disease conditions is still evolving. Evidence is mounting on causal relations of abnormal white matter microstructure and connectivity in a wide range of pediatric neurocognitive and white matter diseases. Hence there is a pressing need for every neuroradiologist to acquire a strong basic knowledge of white matter anatomy and to make an effort to apply this knowledge in routine reporting. INTRODUCTION (Fig. 1). However, the use of specific DTI sequences provides far more detailed and clini- DTI has allowed in vivo demonstration of axonal cally useful information. architecture and connectivity. This technique has set the stage for numerous studies on normal and abnormal connectivity and their role in devel- DIFFUSION TENSOR IMAGING: THE BASICS opmental and acquired disorders. Referencing established white matter anatomy, DTI atlases, Using appropriate magnetic field gradients, and neuroanatomical descriptions, this article diffusion-weighted sequences can be used to summarizes the major white matter anatomy and detect the motion of the water molecules to and related structures relevant to the clinical neurora- from cells. This free movement of the water mole- diologist in daily practice. -

Thalamic Interaction Between the Input and the Output Systems of the Basal Ganglia
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Dadun, University of Navarra Journal of Chemical Neuroanatomy 16 (1999) 187–200 Review Thalamic interaction between the input and the output systems of the basal ganglia Elisa Mengual a, Silvano de las Heras b, Elena Erro a, Jose´ Luis Lanciego a, Jose´ Manuel Gime´nez-Amaya a,* a Departamento de Anatomı´a, Facultad de Medicina, Uni6ersidad de Na6arra, C/ Irunlarrea, 1, 31008 Pamplona, Spain b Departamento de Morfologı´a, Facultad de Medicina, Uni6ersidad Auto´noma de Madrid, 28029 Madrid, Spain Received 6 April 1998; received in revised form 22 October 1998; accepted 14 February 1999 Abstract The striatal return through the thalamus is largely neglected in current studies dealing with basal ganglia function, and its role within this circuitry remains obscure. In this contribution the thalamus is regarded as an important place of interaction between the input and the output organization of the basal ganglia. In support of this idea, a brief overview is provided of some of the most recent findings concerning the thalamus in relation to the basal ganglia circuitry. In particular, we have focused on the thalamostriatal projections themselves, on the output of the basal ganglia to the thalamus and also on the overlapping territories between the thalamic projection of the output nuclei and the thalamostriatal neurons. These data support the existence of several thalamic feedback circuits within the basal ganglia neural system. Finally, some considerations are provided upon the functional significance of these thalamic feedback circuits in the overall organization of the basal ganglia. -
Level 00 Level 01 Level 02 Level 03
Level 00 Level 01 Level 02 Level 03 Level 04 Level 05 Level 06 Level 07 Level 08 Level 09 Level 10 Level 11 Level 12 Level 13 NP::neural plate F::forebrain SP::secondary prosencephalon RSP::rostral secondary prosencephalon POTel::preoptic (impar) telencephalon (preoptic area) POR::preoptic roof plate RSCom::rostral septocommissural area RSComv::ventricular zone of RSCom ACBed::anterior commissure bed RSComm::mantle zone of RSCom MnPOC::median preoptic nucleus, commissural part POA::preoptic alar plate PO::preoptic area LTer::lamina terminalis area LTerv::ventricular zone of LTer LTerm::mantle zone of LTer LTerp::periventricular stratum of the LTer VOLT::vascular organ of the lamina terminalis LTeri::intermediate stratum of the LTer MnPOI::median preoptic nucleus, intermediate part LTers::superficial stratum of the LTer MnPOS::median preoptic nucleus, superficial part PO1::first (dorsal) preoptic domain PO1v::ventricular zone of PO1 PO1m::mantle zone of PO1 PO1p::periventricular stratum of PO1 AVPe::anteroventral periventricular preoptic nucleus RSPO::rostral septopreoptic nucleus PO1i::intermediate stratum of PO1 DMPAi::dorsomedial preoptic area, intermediate part AlPO::alar preoptic nucleus PO1s::superficial stratum of PO1 DMPAs::dorsomedial preoptic area, superficial part PO2::second (ventral) preoptic domain PO2v::ventricular zone of PO2 PO2m::mantle zone of PO2 PO2p::periventricular stratum of PO2 MPOPe::medial preoptic periventricular stratum MPO::medial preoptic nucleus MPOL::lateral part of MPO MPOM::medial part of MPO MPOC::central -
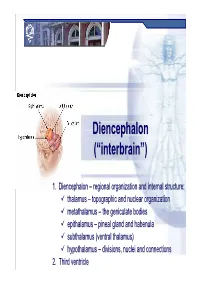
Diencephalondiencephalon ((““Interbraininterbrain ””))
DiencephalonDiencephalon ((““interbraininterbrain ””)) 1. Diencephalon – regional organization and internal structure: thalamus – topographic and nuclear organization metathalamus – the geniculate bodies epithalamus – pineal gland and habenula subthalamus (ventral thalamus) hypothalamus – divisions, nuclei and connections 2. Third ventricle Diencephalon EmbryologicEmbryologic developmentdevelopment Embryonic origin:origin side walls of the prosencephalon (forebrain) Location – at the midline of the brain: caudally – mesencephalon cranially – telencephalon Functions: relay system between sensory input neurons and other parts of the brain works in tandem with the limbic system Prof. Dr. Nikolai Lazarov 2 Diencephalon DiencephalonDiencephalon –– grossgross structurestructure andand partsparts Prof. Dr. Nikolai Lazarov 3 Thalamus ThalamusThalamus –– externalexternal featuresfeatures two egg -shaped lobes of grey matter Gr. θάλαµος = room , chamber third ventricle medially Gr. θάλαµος = room , chamber hypothalamus hypothalamic sulcus Thalamus dorsalis : nuclear complex – 2% of the total brain rostral pole = tuberculum anterius nuclear complex – 2% of the total brain thalami about 80% caudal pole = pulvinar thalami of diencephalic mass (“cushioned seat ”) ~30 mm long interthalamic adhesion ~20 mm wide lamina affixa ~20 mm tall stria terminalis thalami Prof. Dr. Nikolai Lazarov 4 Thalamus ThalamusThalamus –– internalinternal structurestructure internal medullary lamina three major nuclear masses: (medial) – Y-shaped -

Afferents to the Central Nucleus of the Amygdala And
Brain Research 887 (2000) 157±173 www.elsevier.com/locate/bres Research report Afferents to the central nucleus of the amygdala and functional subdivisions of the periaqueductal gray: neuroanatomical substrates for affective behavior Jamespaul Paredes, Ray W. Winters, Neil Schneiderman, Philip M. McCabe* Department of Psychology, University of Miami, P.O. Box 248185, Coral Gables, FL 33124, USA Accepted 19 September 2000 Abstract Evidence suggests the periaqueductal gray (PAG) is involved in the integration of behavioral and autonomic components of affective behavior. Our laboratory has shown that electrical stimulation of the ventrolateral periaqueductal gray (vl PAG) versus the dorsolateral periaqueductal gray (dl PAG), in the rabbit, elicits two distinct behavioral/cardiorespiratory response patterns. Furthermore, evidence suggests that the amygdaloid central nucleus (ACe) may in¯uence cardiovascular activity during emotional states. The purpose of this study was to delineate the topography and determine the origin of forebrain projections to the PAG and the ACe, as well as commonalties and differences in the pattern of afferents. Examination of common afferents may lend insights into their function as components of a forebrain system regulating autonomic activity during emotional states. Separate retrograde tracers were injected into functional subdivisions of the PAG and the ACe in rabbits. PAG injections led to neuronal labeling in numerous cortical regions including the ipsilateral medial prefrontal and insular cortices. Additionally, bilateral labeling was observed in several hypothalamic nuclei including the paraventricular nucleus, the dorsomedial nucleus and the ventromedial nucleus as well as the region lateral to the descending column of the fornix. Sparse labeling was also seen in various basal forebrain regions, thalamic nuclei and amygdaloid nuclei. -

Human Pallidothalamic and Cerebellothalamic Tracts: Anatomical Basis for Functional Stereotactic Neurosurgery
CORE Metadata, citation and similar papers at core.ac.uk Provided by Springer - Publisher Connector Brain Struct Funct (2008) 212:443–463 DOI 10.1007/s00429-007-0170-0 ORIGINAL ARTICLE Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery Marc N. Gallay Æ Daniel Jeanmonod Æ Jian Liu Æ Anne Morel Received: 7 September 2007 / Accepted: 20 December 2007 / Published online: 10 January 2008 Ó The Author(s) 2008 Abstract Anatomical knowledge of the structures to be two tracts in the subthalamic region, (3) the possibility to targeted and of the circuitry involved is crucial in stereo- discriminate between different subthalamic fibre tracts on tactic functional neurosurgery. The present study was the basis of immunohistochemical stainings, (4) correla- undertaken in the context of surgical treatment of motor tions of histologically identified fibre tracts with high- disorders such as essential tremor (ET) and Parkinson’s resolution MRI, and (5) evaluation of the interindividual disease (PD) to precisely determine the course and three- variability of the fibre systems in the subthalamic region. dimensional stereotactic localisation of the cerebellotha- This study should provide an important basis for accurate lamic and pallidothalamic tracts in the human brain. The stereotactic neurosurgical targeting of the subthalamic course of the fibre tracts to the thalamus was traced in the region in motor disorders such as PD and ET. subthalamic region using multiple staining procedures and their entrance into the thalamus determined according to Keywords Basal ganglia Á Thalamus Á Motor disorders Á our atlas of the human thalamus and basal ganglia [Morel Essential tremor Á Parkinson’s disease (2007) Stereotactic atlas of the human thalamus and basal ganglia. -
Thalamus and Limbic System
Thalamus and Limbic system مﻻحظة: .هذا الملف للمراجعة وترتيب المعلومات فقط وليس مرجع للمذاكرة ﻻنه ليست كل المعلومات متضمنة Done by: رند الحميضي Thalamus “found in diencephalon “: is the largest nuclear mass of the body , it is our pathway to the cortex “ last relay site “ , except “ olfaction “ they go directly to the cortex without passing by it. lateral nuclear group , medial , anterior “names only”: Lateral:(L) :Posterior limb of the internal •its projection called the capsule Anterior anterior tubercle. •It lies just behind the end interventricular foramen. Medial: Superior: (s) "3rd ventricle" Lateral has 4 connected to ventricle and surfaces fornix. thalamus of the opposite side by the Massa intermedia. • projection called Pulvinar Posterior • lies above the superior Inferior: (I) colliculus and the lateral & Hypothalamus, end: medial Geniculate bodies anteriorly & Subthalamus posteriorly. Internal medullary lamina: External medullary lamina: Bundle of myelinated (afferent & efferent) fibers. Covers the lateral surface. divide the thalamus into: anterior , It consists of thalamocortical & medial, lateral nuclear groups each corticothalamic fibers one of them contain bunch of nuclei Projection of thalamic nuclei : Name of Nucleus Afferent Efferent Anterior Thalamic Nucleus Mammillary body.” under the Cingulate gyrus, (limbic system) hypothalamus Medial Nucleus or dorso medial Hypothalamus. Frontal &Prefrontal cortex Ventral Anterior Nucleus Globus pallidus body. Premotor cortex. Ventral Lateral Nucleus Dentate Nucleus “cerebellum -

Thalamic Morphometric Changes Induced by First‐Person Action Videogame Training
Received: 16 December 2017 | Revised: 8 October 2018 | Accepted: 2 November 2018 DOI: 10.1111/ejn.14272 RESEARCH REPORT Thalamic morphometric changes induced by first‐person action videogame training Davide Momi1 | Carmelo Smeralda1 | Giulia Sprugnoli1 | Francesco Neri1 | Simone Rossi1,2,3 | Alessandro Rossi1,2,3 | Giorgio Di Lorenzo4,5 | Emiliano Santarnecchi1,3,6 1Siena Brain Investigation & Neuromodulation Lab (Si‐BIN Lab), Neurology and Clinical Neurophysiology Section, Department of Medicine, Surgery and Neuroscience, University of Siena, Siena, Italy 2Human Physiology Section, Department of Medicine, Surgery and Neuroscience, University of Siena, Siena, Italy 3Dipartimento di Scienze Mediche, Chirurgiche e Neuroscienze, Università di Siena, Siena, Italy 4Laboratory of Psychophysiology, Psychiatric Chair, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy 5Psychiatry and Clinical Psychology Unit, Department of Neurosciences, Fondazione Policlinico “Tor Vergata”, Rome, Italy 6Harvard Medical School, Berenson‐Allen Center for Non‐Invasive Brain Stimulation, Beth Israel Deaconess Medical Center, Boston, Massachusetts Correspondence Emiliano Santarnecchi, Harvard Medical Abstract School, Berenson‐Allen Center for Non‐ Cross‐sectional data suggest videogaming as promoting modifications in perceptual Invasive Brain Stimulation, Beth Israel and cognitive skills of players, as well as inducing structural brain changes. However, Deaconess Medical Center, Boston, MA. Email: [email protected] whether such changes are both possible after a systematic gaming exposure, and last beyond the training period, is not known. Here, we originally quantified immediate and long‐lasting cognitive and morphometric impact of a systematic gaming experi- ence on a first‐person shooter (FPS) game. Thirty‐five healthy participants, assigned to a videogaming and a control group, underwent a cognitive assessment and struc- tural magnetic resonance imaging at baseline (T0), immediately post‐gaming (T1) and after 3 months (T2).