Role of DNA Methylation in Adipogenesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A High-Fat/High-Sucrose Diet Induces WNT4 Expression in Mouse Pancreatic Β-Cells
This is “Advance Publication Article” Kurume Medical Journal, 65, 00-00, 2018 Original Article A High-Fat/High-Sucrose Diet Induces WNT4 Expression in Mouse Pancreatic β-cells YAYOI KURITA, TSUYOSHI OHKI, ERI SOEJIMA, XIAOHONG YUAN, SATOMI KAKINO, NOBUHIKO WADA, TOSHIHIKO HASHINAGA, HITOMI NAKAYAMA, JUNICHI TANI, YUJI TAJIRI, YUJI HIROMATSU, KENTARO YAMADA* AND MASATOSHI NOMURA Division of Endocrinology and Metabolism, Department of Internal Medicine, Kurume University School of Medicine, Kurume 830-0011, Japan, *Diabetes Center of Asakura Medical Association Hospital, Asakura 838-0069, Japan Received 12 February 2018, accepted 1 May 2018 J-STAGE advance publication 11 March 2019 Edited by MAKOTO TAKANO Summary: Aims/Introduction: Several lines of evidence suggest that dysregulation of the WNT signaling pathway is involved in the pathogenesis of type 2 diabetes. This study was performed to elucidate the effects of a high-fat/high-sucrose (HF/HS) diet on pancreatic islet functions in relation to modulation of WNT ligand expression in β-cells. Materials and Methods: Mice were fed either standard mouse chow or a HF/HS diet from 8 weeks of age. At 20 weeks of age, intraperitoneal glucose tolerance tests were performed in both groups of mice, followed by euthanasia and isolation of pancreatic islets. WNT-related gene expression in islets and MIN6 cells was measured by quantitative real-time RT-PCR. To explore the direct effects of WNT signals on pancreatic β-cells, MIN6 cells were exposed to recombinant mouse WNT4 protein (rmWNT4) for 48 h, and glucose-induced insulin secre- tion was measured. Furthermore, Wnt4 siRNAs were transfected into MIN6 cells, and cell viability and insulin secretion were measured in control and Wnt4 siRNA-transfected MIN6 cells. -

WNT10A Gene Wnt Family Member 10A
WNT10A gene Wnt family member 10A Normal Function The WNT10A gene is part of a large family of WNT genes, which play critical roles in development starting before birth. These genes provide instructions for making proteins that participate in chemical signaling pathways in the body. Wnt signaling controls the activity of certain genes and regulates the interactions between cells during embryonic development. The protein produced from the WNT10A gene plays a role in the development of many parts of the body. It appears to be essential for the formation of tissues that arise from an embryonic cell layer called the ectoderm. These tissues include the skin, hair, nails, teeth, and sweat glands. Researchers believe that the WNT10A protein is particularly important for the formation and shaping of both baby (primary) teeth and adult ( permanent) teeth. Health Conditions Related to Genetic Changes Hypohidrotic ectodermal dysplasia Several mutations in the WNT10A gene have been found to cause hypohidrotic ectodermal dysplasia, the most common form of ectodermal dysplasia. Starting before birth, ectodermal dysplasias result in the abnormal development of the skin, hair, nails, teeth, and sweat glands. Hypohidrotic ectodermal dysplasia is characterized by a reduced ability to sweat (hypohidrosis), sparse scalp and body hair (hypotrichosis), and several missing teeth (hypodontia) or teeth that are malformed. WNT10A gene mutations account for about 5 percent of all cases of hypohidrotic ectodermal dysplasia. Most of the WNT10A gene mutations associated with hypohidrotic ectodermal dysplasia change single protein building blocks (amino acids) in the WNT10A protein, which impairs its function. The resulting shortage of functional WNT10A protein disrupts Wnt signaling during the development of ectodermal tissues, particularly the teeth. -

Towards an Integrated View of Wnt Signaling in Development Renée Van Amerongen and Roel Nusse*
HYPOTHESIS 3205 Development 136, 3205-3214 (2009) doi:10.1242/dev.033910 Towards an integrated view of Wnt signaling in development Renée van Amerongen and Roel Nusse* Wnt signaling is crucial for embryonic development in all animal Notably, components at virtually every level of the Wnt signal species studied to date. The interaction between Wnt proteins transduction cascade have been shown to affect both β-catenin- and cell surface receptors can result in a variety of intracellular dependent and -independent responses, depending on the cellular responses. A key remaining question is how these specific context. As we discuss below, this holds true for the Wnt proteins responses take shape in the context of a complex, multicellular themselves, as well as for their receptors and some intracellular organism. Recent studies suggest that we have to revise some of messengers. Rather than concluding that these proteins are shared our most basic ideas about Wnt signal transduction. Rather than between pathways, we instead propose that it is the total net thinking about Wnt signaling in terms of distinct, linear, cellular balance of signals that ultimately determines the response of the signaling pathways, we propose a novel view that considers the receiving cell. In the context of an intact and developing integration of multiple, often simultaneous, inputs at the level organism, cells receive multiple, dynamic, often simultaneous and of both Wnt-receptor binding and the downstream, sometimes even conflicting inputs, all of which are integrated to intracellular response. elicit the appropriate cell behavior in response. As such, the different signaling pathways might thus be more intimately Introduction intertwined than previously envisioned. -

Wnt4/B2catenin Signaling in Medullary Kidney Myofibroblasts
BASIC RESEARCH www.jasn.org Wnt4/b2Catenin Signaling in Medullary Kidney Myofibroblasts † †‡ | Derek P. DiRocco,* Akio Kobayashi,* Makoto M. Taketo,§ Andrew P. McMahon, and †‡ Benjamin D. Humphreys* *Renal Division, Brigham and Women’s Hospital, Boston, Massachusetts; †Harvard Medical School, Boston, Massachusetts; ‡Harvard Stem Cell Institute, Cambridge, Massachusetts; §Department of Pharmacology, Graduate School of Medicine, Kyoto University, Yoshida-Konoé-cho, Sakyo, Kyoto, Japan; and |Department of Stem Cell Biology and Regenerative Medicine, Eli and Edythe Broad-CIRM Center for Regenerative Medicine and Stem Cell Research, Keck School of Medicine of the University of Southern California, Los Angeles, California ABSTRACT Injury to the adult kidney induces a number of developmental genes thought to regulate repair, including Wnt4. During kidney development, early nephron precursors and medullary stroma both express Wnt4, where it regulates epithelialization and controls smooth muscle fate, respectively. Expression patterns and roles for Wnt4 in the adult kidney, however, remain unclear. In this study, we used reporters, lineage analysis, and conditional knockout or activation of the Wnt/b-catenin pathway to investigate Wnt4 in the adult kidney. Proliferating, medullary, interstitial myofibroblasts strongly expressed Wnt4 during renal fibrosis, whereas tubule epithelia, except for the collecting duct, did not. Exogenous Wnt4 drove myofi- broblast differentiation of a pericyte-like cell line, suggesting that Wnt4 might regulate pericyte-to-myo- fibroblast transition through autocrine signaling. However, conditional deletion of Wnt4 in interstitial cells did not reduce myofibroblast proliferation, cell number, or myofibroblast gene expression during fibrosis. Because the injured kidney expresses multiple Wnt ligands that might compensate for the absence of Wnt4, we generated a mouse model with constitutive activation of canonical Wnt/b-catenin signaling in interstitial pericytes and fibroblasts. -

Induction of Apoptosis Increases Expression of Non-Canonical WNT Genes in Myeloid Leukemia Cell Lines
1563-1569 7/11/07 19:04 Page 1563 ONCOLOGY REPORTS 18: 1563-1569, 2007 Induction of apoptosis increases expression of non-canonical WNT genes in myeloid leukemia cell lines HAKKI OGUN SERCAN1, MELEK PEHLIVAN1, OZLENEN SIMSEK1, HALIL ATES2 and ZEYNEP SERCAN1 Depatments of 1Medical Biology and Genetics, 2Hematology/Oncology, Dokuz Eylul University Faculty of Medicine, Izmir, Turkey Received May 3, 2007; Accepted July 20, 2007 Abstract. With the aim of determining the differential and cyclin D. The presence or exclusion of co-receptors (low expression of WNT and FZD genes, before and after density lipoprotein receptor-related protein 5 and 6-LRP5/6) induction of apoptosis in BCR-ABL positive cells, we treated or secreted Wnt antagonists (soluble frizzled related proteins the myeloid cell line K562 and control cell line HL60 with - sFRPs- and Dickkopf proteins) increases the complexity of imatinib mesylate and etoposide, and analyzed relative the molecular mechanisms underlying cellular outcomes. mRNA expression levels of WNT, FZD and sFRP genes Non-canonical pathway activation is independent of ß- under normal and apoptotic conditions by real-time RT-PCR. catenin. Two non-canonical pathways have also been defined: We observed marked increase in mRNA levels of FZD4, the planar cell polarity (PCP) and the Wnt/Ca2+ pathways (3-6). FZD5, FZD7 and WNT5b, correlating with apoptotic activity There is an overlap of proposed Wnt and Fzd proteins and and independent of the agent or cell line used. Our results secondary messengers participating in these non-canonical suggest the involvement of non-canonical Wnt signaling in pathways, complicating further the interpretation of experi- executing programmed cell death in myeloid cell lines. -

Novel Missense Mutations in the AXIN2 Gene Associated with Non
a r c h i v e s o f o r a l b i o l o g y 5 9 ( 2 0 1 4 ) 3 4 9 – 3 5 3 Available online at www.sciencedirect.com ScienceDirect journal homepage: http://www.elsevier.com/locate/aob Novel missense mutations in the AXIN2 gene associated with non-syndromic oligodontia a,1 a,1 b a Singwai Wong , Haochen Liu , Baojing Bai , Huaiguang Chang , c,d e a, a, Hongshan Zhao , Yixiang Wang , Dong Han **, Hailan Feng * a Department of Prosthodontics, Peking University School and Hospital of Stomatology, Beijing, China b Department of Prosthodontics, Beijing Stomatology Hospital, Beijing, China c Department of Medical Genetics, Peking University Health Science Center, Beijing, China d Peking University Center for Human Disease Genomics, Peking University Health Science Center, Beijing, China e Central Laboratory, Peking University School and Hospital of Stomatology, Beijing, China a r t i c l e i n f o a b s t r a c t Article history: Objective: Oligodontia, which is the congenital absence of six or more permanent teeth Accepted 23 December 2013 excluding third molars, may contribute to masticatory dysfunction, speech alteration, aesthetic problems and malocclusion. To date, mutations in EDA, AXIN2, MSX1, PAX9, Keywords: WNT10A, EDAR, EDARADD, NEMO and KRT 17 are known to associate with non-syndromic oligodontia. The aim of the study was to search for AXIN2 mutations in 96 patients with non- Non-syndromic oligodontia syndromic oligodontia. Mutation screening AXIN2 Design: We performed mutation analysis of 10 exons of the AXIN2 gene in 96 patients with isolated non-syndromic oligodontia. -

The WNT10A Gene in Ectodermal Dysplasias and Selective Tooth Agenesis Gabriele Mues,1* John Bonds,1 Lilin Xiang,1 Alexandre R
RESEARCH ARTICLE The WNT10A Gene in Ectodermal Dysplasias and Selective Tooth Agenesis Gabriele Mues,1* John Bonds,1 Lilin Xiang,1 Alexandre R. Vieira,2 Figen Seymen,3 Ophir Klein,4,5,6 and Rena N. D’Souza1 1Department of Biomedical Sciences, Texas A&M University-HSC Baylor College of Dentistry, Dallas, Texas 2Department of Oral Biology, University of Pittsburgh School of Dental Medicine, Pittsburgh, Pennsylvania 3University of Istanbul Faculty of Dentistry, Istanbul, Turkey 4Department of Orofacial Sciences, University of California San Francisco, San Francisco, California 5Department of Pediatrics, University of California San Francisco, San Francisco, California 6Institute for Human Genetics and Regeneration Medicine, University of California San Francisco, San Francisco, California Manuscript Received: 8 October 2013; Manuscript Accepted: 30 January 2014 Mutations in the WNT10A gene were first detected in the rare syndrome odonto-onycho-dermal dysplasia (OODD, How to Cite this Article: OMIM257980) but have now also been found to cause about Mues G, Bonds J, Xiang L, Vieira AR, 35–50% of selective tooth agenesis (STHAG4, OMIM150400), a Seymen F, Klein O, D’Souza RN. 2014. common disorder that mostly affects the permanent dentition. The WNT10A gene in ectodermal In our random sample of tooth agenesis patients, 40% had at least dysplasias and selective tooth agenesis. one mutation in the WNT10A gene. The WNT10A Phe228Ile variant alone reached an allele frequency of 0.21 in the tooth Am J Med Genet Part A 164A:2455–2460. agenesis cohort, about 10 times higher than the allele frequency reported in large SNP databases for Caucasian populations. Patients with bi-allelic WNT10A mutations have severe tooth syndrome (OMIM 224750) which additionally features eyelid cysts agenesis while heterozygous individuals are either unaffected or and predisposition to adnexal skin tumors. -
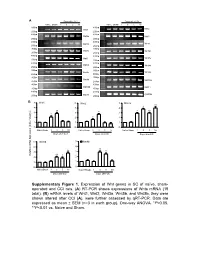
Supplementary Figure 1. Expression of Wnt Genes in SC of Naïve
A Days after CCI Days after CCI Naive Sham 1 3 5 10 Naive Sham 1 3 5 10 500bp 400bp Wnt1 Wnt2 250bp 250bp 400bp 400bp Wnt2b Wnt3 250bp 250bp 400bp 400bp Wnt3a Wnt4 250bp 250bp 300bp 400bp Wnt5a Wnt5b 200bp 250bp 400bp 400bp Wnt6 Wnt7a 250bp 250bp 400bp 400bp Wnt7b Wnt8a 250bp 250bp 400bp 350bp Wnt8b Wnt9a 250bp 200bp 400bp 400bp Wnt9b Wnt10a 250bp 250bp 400bp 400bp Wnt10b Wnt11 250bp 250bp 400bp 400bp Wnt16 GAPDH 250bp 250bp B 5 Wnt1 5 Wnt2 5 Wnt3a ** 4 4 4 * ** ** 3 3 * 3 * * 2 2 * 2 1 1 1 0 0 0 NaïveSham 1 3 5 10 Naïve Sham 1 3 5 10 Naïve Sham 1 3 5 10 Days after CCI Days after CCI Days after CCI 5 Wnt5b 5 Wnt8b 4 4 ** 3 ** 3 ** Relative mRNA Expression (fold of change) ** 2 * 2 1 1 0 0 NaïveSham 1 3 5 10 NaïveSham 1 3 5 10 Days after CCI Days after CCI Supplementary Figure 1 A B Negative Weak Moderate Strong Negative Weak Moderate Strong Large-sized cells 100% 100% 80% 80% 60% 60% 40% 20% 40% 0% 20% Sham CCI-1d CCI-14d Medium-sized cells Wnt3a immunoreactivity cells 0% 100% CGRP(+) IB4(+) 80% Small cells 60% 40% 20% 0% Sham CCI-1d CCI-14d Wnt3a immunoreactivity cells Small cells 100% 80% 60% 40% 20% 0% Sham CCI-1d CCI-14d Fig. S2 4 Fz1 4 Fz3 4 Fz4 4 Fz5 ** 3 3 ** 3 3 2 ** 2 2 ** 2 * 1 1 1 1 0 0 0 0 NaïveSham 1 5 10 NaïveSham 1 5 10 Naïve Sham 1 5 10 NaïveSham 1 5 10 Days after CCI Days after CCI Days after CCI Days after CCI 4 Fz6 4 Fz7 4 Fz8 4 Fz9 (Fold of Change) of (Fold 3 3 3 ** 3 Relative mRNA Expression Expression mRNA Relative ** 2 2 2 ** 2 * 1 1 1 1 0 0 0 0 NaïveSham 1 5 10 NaïveSham 1 5 10 Naïve Sham 1d 5d 10d NaïveSham 1 -

Variability in Dentofacial Phenotypes in Four Families with WNT10A Mutations
European Journal of Human Genetics (2014) 22, 1063–1070 & 2014 Macmillan Publishers Limited All rights reserved 1018-4813/14 www.nature.com/ejhg ARTICLE Variability in dentofacial phenotypes in four families with WNT10A mutations Christian P Vink1, Charlotte W Ockeloen2,3, Sietske ten Kate2, David A Koolen2, Johannes Kristian Ploos van Amstel4, Anne-Marie Kuijpers-Jagtman1,3, Celeste C van Heumen3,5, Tjitske Kleefstra2,3 and Carine EL Carels*,1,3,6,7 This article describes the inter- and intra-familial phenotypic variability in four families with WNT10A mutations. Clinical characteristics of the patients range from mild to severe isolated tooth agenesis, over mild symptoms of ectodermal dysplasia, to more severe syndromic forms like odonto-onycho-dermal dysplasia (OODD) and Scho¨pf–Schulz–Passarge syndrome (SSPS). Recurrent WNT10A mutations were identified in all affected family members and the associated symptoms are presented with emphasis on the dentofacial phenotypes obtained with inter alia three-dimensional facial stereophotogrammetry. A comprehensive overview of the literature regarding WNT10A mutations, associated conditions and developmental defects is presented. We conclude that OODD and SSPS should be considered as variable expressions of the same WNT10A genotype. In all affected individuals, a dished-in facial appearance was observed which might be helpful in the clinical setting as a clue to the underlying genetic etiology. European Journal of Human Genetics (2014) 22, 1063–1070; doi:10.1038/ejhg.2013.300; published online 8 January 2014 Keywords: ectodermal dysplasia; odonto-onycho-dermal dysplasia; Scho¨pf–Schulz–Passarge syndrome; tooth agenesis; WNT10A INTRODUCTION including homozygous, compound heterozygous and heterozygous Tooth agenesis (TA) is the most common, often heritable, develop- mutations. -

Six Key Genes in the Ectodermal Dysplasias EDA, EDAR, EDARADD, WNT10A, IKBKG (Aka NEMO) and TP63
Six Key Genes in the Ectodermal Dysplasias EDA, EDAR, EDARADD, WNT10A, IKBKG (aka NEMO) and TP63 One question we are often asked is about the differences between the key genes involved in the different types of ectodermal dysplasia (ED). There are many different types of ED and many different genes can be involved. There is a small number of key genes that are most typically involved as well as many rare genes. All these genes are present in everyone but changes in the code of that gene can cause problems if the code change alters the genetic instruction. So, when a particular gene is said to be involved in one individual with one specific type of ED, it means that a change in that gene is responsible for their form of ED. In families where more than one family member has ED they almost always share the same change in the same gene. Genes A gene can be thought of as an instruction – as in a recipe - for how the body should assemble itself and how it can function. Genes consist of long runs (sequences) of the four different building blocks of DNA (Deoxyribose Nucleic Acid) A, C, G and T. The genes come in matching pairs, with one gene of each pair coming from each parent. The 20,000 or so different types of gene are all made of the DNA (those long strings of A, C, G and T) and are carried in the 23 chromosomes present in the sperm and the 23 chromosomes present in the egg that went to make each of us, with our 46 chromosomes. -

Expression and the Clinical Significance of Wnt10a and Wnt10b in Endometrial Cancer Are Associated with the Wnt/Β-Catenin Pathway
ONCOLOGY REPORTS 29: 507-514, 2013 Expression and the clinical significance of Wnt10a and Wnt10b in endometrial cancer are associated with the Wnt/β-catenin pathway HONGMAN CHEN1,2, YINGMEI WANG1 and FENGXIA XUE1 1Department of Gynecology and Obstetrics, Tianjin Medical University General Hospital, Tianjin 300052; 2Department of Gynecology and Obstetrics, The Fifth Central Hospital of Tianjin, Tianjin 300450, P.R. China Received July 31, 2012; Accepted September 24, 2012 DOI: 10.3892/or.2012.2126 Abstract. To determine the role played by the Wnt/β-catenin tosis, primarily through the activation of the Wnt/β-catenin signaling pathway in the development of endometrial cancer pathway. The role played by Wnt10a in EC, however, still (EC), we examined the expression of Wnt10a and Wnt10b requires further investigation. in EC tissues and the correlation between their expression. Furthermore, the associations between these two proteins and Introduction the clinicopathological characteristics and prognosis of EC were also evaluated. In our search of alternative mechanisms, Endometrial cancer (EC) is one of the most common gyneco- we investigated the impact of Wnt10b on proliferation and logic malignancies in developed countries. In the US alone, apoptosis of EC cells. Western blotting, 3-(4, 5-dimethylthiazol- 46,470 new cases and 8,120 deaths from EC were estimated in 2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and flow 2011 (1). In China, the incidence of this malignancy has also cytometry were used to evaluate the expression of Wnt10b and been observed to increase rapidly, threatening women's health some key proteins of the Wnt/β-catenin pathway, proliferation (2). -

EDA, EDAR, EDARADD and WNT10A Allelic Variants in Patients With
Martínez-Romero et al. Orphanet Journal of Rare Diseases (2019) 14:281 https://doi.org/10.1186/s13023-019-1251-x RESEARCH Open Access EDA, EDAR, EDARADD and WNT10A allelic variants in patients with ectodermal derivative impairment in the Spanish population María Carmen Martínez-Romero1,2, María Juliana Ballesta-Martínez3,4, Vanesa López-González3,4, María José Sánchez-Soler3,4, Ana Teresa Serrano-Antón3, María Barreda-Sánchez4, Lidya Rodriguez-Peña3, María Teresa Martínez-Menchon5, José Frías-Iniesta5, Paloma Sánchez-Pedreño5, Pablo Carbonell-Meseguer1, Guillermo Glover-López1, Encarna Guillén-Navarro6,7* and GIEDE (Spanish multidisciplinary research group for ectodermal dysplasia) Abstract Background: Ectodermal dysplasias (ED) are a group of genetic conditions affecting the development and/or homeostasis of two or more ectodermal derivatives. An attenuated phenotype is considered a non-syndromic trait when the patient is affected by only one impaired ectodermal structure, such as in non-syndromic tooth agenesis (NSTA) disorder. Hypohidrotic ectodermal dysplasia (HED) is the most highly represented ED. X-linked hypohidrotic ectodermal dysplasia (XLHED) is the most common subtype, with an incidence of 1/50,000–100,000 males, and is associated with the EDA gene (Xq12-q13.1); the dominant and recessive subtypes involve the EDAR (2q13) and EDARADD (1q42.3) genes, respectively. The WNT10A gene (2q35) is associated more frequently with NSTA. Our goal was to determine the mutational spectrum in a cohort of 72 Spanish patients affected by one or more ectodermal derivative impairments referred to as HED (63/72) or NSTA (9 /72) to establish the prevalence of the allelic variants of the four most frequently associated genes.