On Norfolk Island, South Pacific
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
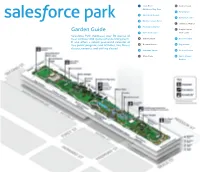
Salesforce Park Garden Guide
Start Here! D Central Lawn Children’s Play Area Garden Guide6 Palm Garden 1 Australian Garden Start Here! D Central Lawn Salesforce Park showcases7 California over Garden 50 species of Children’s Play Area 2 Mediterraneantrees and Basin over 230 species of understory plants. 6 Palm Garden -ã ¼ÜÊ ÊăØÜ ØÊèÜãE úØƀØÊèÃJapanese Maples ¼ÃØ Ê¢ 1 Australian Garden 3 Prehistoric¢ØÕ輫ÕØÊ£ØÂÜÃã«ó«ã«Üŧ¼«¹ĆãÃÜÜ Garden 7 California Garden ¼ÜÜÜŧÊÃØãÜŧÃØ¢ã«Ã£¼ÜÜÜũF Amphitheater Garden Guide 2 Mediterranean Basin 4 Wetland Garden Main Lawn E Japanese Maples Salesforce Park showcases over 50 species of 3 Prehistoric Garden trees and over 230 species of understory plants. A Oak Meadow 8 Desert Garden F Amphitheater It also offers a robust year-round calendar of 4 Wetland Garden Main Lawn free public programs and activities, like fitness B Bamboo Grove 9 Fog Garden Desert Garden classes, concerts, and crafting classes! A Oak Meadow 8 5 Redwood Forest 10 Chilean Garden B Bamboo Grove 9 Fog Garden C Main Plaza 11 South African 10 Chilean Garden Garden 5 Redwood Forest C Main Plaza 11 South African Garden 1 Children’s Australian Play Area Garden ABOUT THE GARDENS The botanist aboard the Endeavor, Sir Joseph Banks, is credited with introducing many plants from Australia to the western world, and many This 5.4 acre park has a layered soil system that plants today bear his name. balances seismic shifting, collects and filters storm- water, and irrigates the gardens. Additionally, the soil Native to eastern Australia, Grass Trees may grow build-up and dense planting help offset the urban only 3 feet in 100 years, and mature plants can be heat island effect by lowering the air temperature. -

TAG Operational Structure
PARROT TAXON ADVISORY GROUP (TAG) Regional Collection Plan 5th Edition 2020-2025 Sustainability of Parrot Populations in AZA Facilities ...................................................................... 1 Mission/Objectives/Strategies......................................................................................................... 2 TAG Operational Structure .............................................................................................................. 3 Steering Committee .................................................................................................................... 3 TAG Advisors ............................................................................................................................... 4 SSP Coordinators ......................................................................................................................... 5 Hot Topics: TAG Recommendations ................................................................................................ 8 Parrots as Ambassador Animals .................................................................................................. 9 Interactive Aviaries Housing Psittaciformes .............................................................................. 10 Private Aviculture ...................................................................................................................... 13 Communication ........................................................................................................................ -

Foraging Ecology of the World's Only
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. FORAGING ECOLOGY OF THE WORLD’S ONLY POPULATION OF THE CRITICALLY ENDANGERED TASMAN PARAKEET (CYANORAMPHUS COOKII), ON NORFOLK ISLAND A thesis presented in partial fulfilment of the requirements for the degree of Master of Science in Conservation Biology at Massey University, Auckland, New Zealand. Amy Waldmann 2016 The Tasman parakeet (Cyanoramphus cookii) Photo: L. Ortiz-Catedral© ii ABSTRACT I studied the foraging ecology of the world’s only population of the critically endangered Tasman parakeet (Cyanoramphus cookii) on Norfolk Island, from July 2013 to March 2015. I characterised, for the first time in nearly 30 years of management, the diversity of foods consumed and seasonal trends in foraging heights and foraging group sizes. In addition to field observations, I also collated available information on the feeding biology of the genus Cyanoramphus, to understand the diversity of species and food types consumed by Tasman parakeets and their closest living relatives as a function of bill morphology. I discuss my findings in the context of the conservation of the Tasman parakeet, specifically the impending translocation of the species to Phillip Island. I demonstrate that Tasman parakeets have a broad and flexible diet that includes seeds, fruits, flowers, pollen, sori, sprout rhizomes and bark of 30 native and introduced plant species found within Norfolk Island National Park. Dry seeds (predominantly Araucaria heterophylla) are consumed most frequently during autumn (81% of diet), over a foraging area of ca. -

Approaching the Prehistory of Norfolk Island
© Copyright Australian Museum, 2001 Records of the Australian Museum, Supplement 27 (2001): 1–9. ISBN 0 7347 2305 9 Approaching the Prehistory of Norfolk Island ATHOLL ANDERSON1 AND PETER WHITE2 1 Department of Archaeology & Natural History, Research School of Pacific and Asian Studies, Australian National University, Canberra ACT 0200, Australia [email protected] 2 Archaeology, University of Sydney, Sydney NSW 2006, Australia [email protected] ABSTRACT. Norfolk Island, on the northeast edge of the Tasman Sea, is of volcanic origin and moderate height. A humid, forested subtropical landmass, it had a diverse range of natural resources, including some food plants such as Cyathea, forest birds such as pigeon and parrot species and substantial colonies of seabirds, notably boobies and procellariids. Its shoreline had few shellfish, but the coastal waters were rich in fish, of which Lethrinids were especially abundant. The island had no inhabitants when discovered by Europeans in A.D. 1774. It was settled by them in A.D. 1788. From the eighteenth century discovery of feral bananas and then of stone adzes, knowledge of the prehistory of Norfolk Island has developed over a very long period. Collections of stone tools seemed predominantly East Polynesian in orientation, but Melanesian sources could not be ruled out. Research on fossil bone deposits established the antiquity of the human commensal Rattus exulans as about 800 B.P. but no prehistoric settlement site was known until one was discovered in 1995 at Emily Bay during the Norfolk Island Prehistory Project. ANDERSON, ATHOLL, AND PETER WHITE, 2001a. Approaching the prehistory of Norfolk Island. -

THE Distrffiution of the AUSTRALIAN PSITTACINES (Order PSITTACIFORMES: Parrots, Cockatoos, Etc.)
THE S.A,' ORNITHOLOGIST 3 THE DISTRffiUTION OF THE AUSTRALIAN PSITTACINES (Order PSITTACIFORMES: Parrots, Cockatoos, etc.) by ALAN LENDON, Adelaide This paper is the culmination of many east-central Queensland and of the Paradise years of documentation of personal obser- Parrot in the vicinity of the Mitchell River. vations and of published records especially those in The Emu and The South' Australian ORDER: PSITTACIFORMES: PARROTS, Ornithologist, of the distribution of the Aus COCKATOOS, ETC. tralian members of the order Psittaciformes. FAMILY TRICHOGLOSSIDAE: LORIKEETS An attempt has been made to collate this in 254 TRICHOGLOSSUS MOLUCCANUS formation with the records of the specimens RAINBOW (BLUE MOUNTAIN) LORIKEET in the various Australian Museums, thanks Checklist distribution-E.A.,- S.A., T. to the courtesy of their Directors, and with Eastern Australia is best divided into the communicated observations of numerous States. In Queensland, there are records field workers, far too numerous to mention from some Torres Strait islands and from individually. Great reliance has been placed all of Cape York Peninsula and thence down on the various regional textbooks of Aus the whole of eastern Queensland without ever tralian birds, particularly Birds of Western going much further west than the limits of Australia by Serventy and WhitteIl, Tasma the Great Dividing Range. It is appreciated nian Birds by Sharland, A H andlist of the that there is considerable overlap of the range Birds of Victoria by Wheeler, A Handlist of of this and the next species at the base of the Birds of New South Wales by McGill, Cape York Peninsula. List of Northern Territory Birds by Storr, and In New South Wales, the recorded distri the papers appearing in The South Australian bution is again limited to the Great Dividing Ornithologist by Terrill and Rix and by Range and to the east thereof and but rarely Condon. -

TAXON:Rhopalostylis Baueri SCORE:-2.0 RATING:Low Risk
TAXON: Rhopalostylis baueri SCORE: -2.0 RATING: Low Risk Taxon: Rhopalostylis baueri Family: Arecaceae Common Name(s): Norfolk Island palm Synonym(s): Areca baueri Hook. f. ex Lem. Eora(basionym) baueri (H. Wendl. & Drude) O. F. RhopalostylisCook cheesemanii Becc. ex Cheeseman Assessor: No Assessor Status: Assessor Approved End Date: WRA Score: -2.0 Designation: L Rating: Low Risk Keywords: Subtropical Palm, Unarmed, Shade-tolerant, Thicket-forming, Bird-dispersed Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 n Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 y outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 n 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) n 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed n=0, y = 2*multiplier (see Appendix 2) n 305 Congeneric weed n=0, y = 1*multiplier -

Anson Bay Reserve 2018 - 2028
PLAN OF MANAGEMENT ANSON BAY RESERVE 2018 - 2028 Anson Bay Reserve Plan of Management 2019 - 2029 Page 2 of 37 Table of Contents 1 INTRODUCTION ........................................................................................................................................ 5 1.1 RESERVE DESCRIPTION ................................................................................................................................. 5 1.2 PUBLIC CONSULTATION AND PLANNING FRAMEWORK ........................................................................................ 5 1.3 HERITAGE LISTING ....................................................................................................................................... 7 2 SIGNIFICANCE OF ANSON BAY RESERVE ................................................................................................... 8 2.1 GEOLOGY AND LANDFORM ............................................................................................................................ 8 2.2 FLORA ....................................................................................................................................................... 9 2.2.1 Significant Plant Species .................................................................................................................. 10 2.3 FAUNA .................................................................................................................................................... 11 2.4 CULTURAL HERITAGE ................................................................................................................................ -

Polynesian Plant Introductions in the Southwest Pacific: Initial Pollen Evidence from Norfolk Island
© Copyright Australian Museum, 2001 Records of the Australian Museum, Supplement 27 (2001): 123–134. ISBN 0 7347 2305 9 Polynesian Plant Introductions in the Southwest Pacific: Initial Pollen Evidence from Norfolk Island MIKE K. MACPHAIL, GEOFFREY S. HOPE AND ATHOLL ANDERSON Department of Archaeology & Natural History, Research School of Pacific and Asian Studies, Australian National University, Canberra ACT 0200, Australia [email protected] [email protected] [email protected] ABSTRACT. Thick organic swamp sediments, buried under land fill on Kingston Common, preserves evidence of the Norfolk Island flora and vegetation back to the middle Holocene and probably much earlier times in the Late Quaternary. These sediments provide (1) a bench mark against which the impact of humans on the flora and vegetation of a long-isolated island can be assessed and (2) a means of determining whether particular plant genera and species are introduced or native to the island. Although sediments contemporary with Polynesian occupation about 800 years ago were destroyed by European draining and cultivation of the swamp during the early nineteenth century, the pollen data indicate that New Zealand flax (Phormium tenax) was introduced to Norfolk Island by Polynesians. Other putative exotics such as Ti (Cordyline), a bull-rush (Typha orientalis) and, less certain, herbs such as the sow thistle (Sonchus oleraceus), were part of the native flora long before the earliest recorded Polynesian settlement. Wildfires have been part of the landscape ecology of Norfolk Island since at least the middle Holocene. MACPHAIL, MIKE K., GEOFFREY S. HOPE AND ATHOLL ANDERSON, 2001. -

Rhopalostylis Sapida
Rhopalostylis sapida COMMON NAME Nikau palm SYNONYMS None FAMILY Arecaceae AUTHORITY Rhopalostylis sapida H.Wendl. et Drude FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes ENDEMIC GENUS No ENDEMIC FAMILY No STRUCTURAL CLASS Trees & Shrubs - Monocotyledons NVS CODE RHOSAP CHROMOSOME NUMBER Whareroa Farm, Paekakariki. Apr 2011. 2n = 32 Photographer: Jeremy Rolfe CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened BRIEF DESCRIPTION Palm to 15m tall with a ringed trunk and 3m long erect leaves inhabiting lowland forest south to Okarito and Banks Peninsula and the Chatham Islands. Leaves with multiple narrow leaflets to 1m long closely-spaced along central stem. Flowers pinkish, in multiple spikes at the top of trunk. Fruit red. DISTRIBUTION Endemic. North Island, South Island from Marlborough Sounds and Nelson south to Okarito in the west and Banks Peninsula in the east. Also on Chatham and Pitt Islands. However Chatham Islands plants have adistinct juveniel form, larger fruits, and thicker indumentum on the fronds. HABITAT Trunk of nikau. Photographer: Wayne Bennett Primarily a species of coastal to lowland forest in the warmer parts of New Zealand. FEATURES Trunk up to 15 m, stout, covered in grey-green leaf scars, otherwise green. Crownshaft 0.6(-1) m long, dark green, smooth, bulging. Fronds up to 3 m long; leaflets to 1 m, closely set (sometimes over lapping), ascending. Spathes c.300 x 150 mm., between pink and yellow, caducous. Inflorescence shortly stalked, with many branches, 200-400 mm long. Flowers sessile, unisexual, tightly packed, lilac to pink. Males in pairs, caducous, stamens 6. -

The Island Rule and Its Application to Multiple Plant Traits
The island rule and its application to multiple plant traits Annemieke Lona Hedi Hendriks A thesis submitted to the Victoria University of Wellington in partial fulfilment of the requirements for the degree of Master of Science in Ecology and Biodiversity Victoria University of Wellington, New Zealand 2019 ii “The larger the island of knowledge, the longer the shoreline of wonder” Ralph W. Sockman. iii iv General Abstract Aim The Island Rule refers to a continuum of body size changes where large mainland species evolve to become smaller and small species evolve to become larger on islands. Previous work focuses almost solely on animals, with virtually no previous tests of its predictions on plants. I tested for (1) reduced floral size diversity on islands, a logical corollary of the island rule and (2) evidence of the Island Rule in plant stature, leaf size and petiole length. Location Small islands surrounding New Zealand; Antipodes, Auckland, Bounty, Campbell, Chatham, Kermadec, Lord Howe, Macquarie, Norfolk, Snares, Stewart and the Three Kings. Methods I compared the morphology of 65 island endemics and their closest ‘mainland’ relative. Species pairs were identified. Differences between archipelagos located at various latitudes were also assessed. Results Floral sizes were reduced on islands relative to the ‘mainland’, consistent with predictions of the Island Rule. Plant stature, leaf size and petiole length conformed to the Island Rule, with smaller plants increasing in size, and larger plants decreasing in size. Main conclusions Results indicate that the conceptual umbrella of the Island Rule can be expanded to plants, accelerating understanding of how plant traits evolve on isolated islands. -

Three Rare Parrots Added to Appendix I of CITES !
PsittaScene In this Issue: Three Rare Parrots Added To Appendix I of CITES ! Truly stunning displays PPsittasitta By JAMIE GILARDI In mid-October I had the pleasure of visiting Bolivia with a group of avid parrot enthusiasts. My goal was to get some first-hand impressions of two very threatened parrots: the Red-fronted Macaw (Ara rubrogenys) and the Blue-throated Macaw (Ara SceneScene glaucogularis). We have published very little about the Red-fronted Macaw in PsittaScene,a species that is globally Endangered, and lives in the foothills of the Andes in central Bolivia. I had been told that these birds were beautiful in flight, but that Editor didn't prepare me for the truly stunning displays of colour we encountered nearly every time we saw these birds. We spent three days in their mountain home, watching them Rosemary Low, fly through the valleys, drink from the river, and eat from the trees and cornfields. Glanmor House, Hayle, Cornwall, Since we had several very gifted photographers on the trip, I thought it might make a TR27 4HB, UK stronger impression on our readers to present the trip in a collection of photos. CONTENTS Truly stunning displays................................2-3 Gold-capped Conure ....................................4-5 Great Green Macaw ....................................6-7 To fly or not to fly?......................................8-9 One man’s vision of the Trust..................10-11 Wild parrot trade: stop it! ........................12-15 Review - Australian Parrots ..........................15 PsittaNews ....................................................16 Review - Spix’s Macaw ................................17 Trade Ban Petition Latest..............................18 WPT aims and contacts ................................19 Parrots in the Wild ........................................20 Mark Stafford Below: A flock of sheep being driven Above: After tracking the Red-fronts through two afternoons, we across the Mizque River itself by a found that they were partial to one tree near a cornfield - it had sprightly gentleman. -

To Nest Or Not to Nest How to Buy Breeding Stock Crimson Rosella
VOL 33 NO 05 Red-tailed Amazons, bees and drones To nest or not to nest Crimson Rosella breeding Information How To Buy Breeding Stock CONTENTS 3 VoLume 35 / Number 03 Editorial 4 Red-tailed Amazons, bees and drones The Red-tailed Amazons nest in forest of the low-lying islands and neighbouring coastal plains, where they face diverse challenges to reproduce successfully. 6 PVSA NUUS / PASA NEWS PASA well-known Focus /FOKUS magazine has been discontinued, as of this edition Avizandum will be the printed media PASA will be using to update its members. 8 TO NEST OR NOT TO NEST » p.8 What started as a project to build more capacity for breeding stock ended up in the building of a display Aviary in front of the breeding collection, to enhance the garden. 10 Breeding by parents 12 Crimson Rosella breeding Information Commonly found in gardens and mountain forests,they soon migrated to the surrounding regions.With such beautiful crimson plumage,they became a favourite companionbirds across the world. 15 Q&A on Dr Henriëtte van der Zwan Avizandum is excited to announce the launch of a new column on genetics. Most bird breeders struggle with this topic. We asked dr. Henriëtte van der Zwan, a researcher in avian » p.16 genetics to shed some light on it. 16 Shabby Fufu lifestyle Farm, Plettenberg Bay Irene Passion being waterfowl, there are Black-necked Swans, Black Swans as well as 10 different species of duck and geese. These are bred in all earnest. 18 How To Buy Breeding Stock For the beginner, trying to make the right decisions concerning the purchase of breeding stock is like jumping into the great unknown.