Auditory Neuropathy Spectrum Disorders: from Diagnosis to Treatment: Literature Review and Case Reports
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pathophysiological Mechanisms and Functional Hearing Consequences of Auditory Neuropathy
doi:10.1093/brain/awv270 BRAIN 2015: 138; 3141–3158 | 3141 REVIEW ARTICLE Pathophysiological mechanisms and functional hearing consequences of auditory neuropathy Gary Rance1 and Arnold Starr2 The effects of inner ear abnormality on audibility have been explored since the early 20th century when sound detection measures were first used to define and quantify ‘hearing loss’. The development in the 1970s of objective measures of cochlear hair cell Downloaded from function (cochlear microphonics, otoacoustic emissions, summating potentials) and auditory nerve/brainstem activity (auditory brainstem responses) have made it possible to distinguish both synaptic and auditory nerve disorders from sensory receptor loss. This distinction is critically important when considering aetiology and management. In this review we address the clinical and pathophysiological features of auditory neuropathy that distinguish site(s) of dysfunction. We describe the diagnostic criteria for: (i) presynaptic disorders affecting inner hair cells and ribbon synapses; (ii) postsynaptic disorders affecting unmyelinated auditory http://brain.oxfordjournals.org/ nerve dendrites; (iii) postsynaptic disorders affecting auditory ganglion cells and their myelinated axons and dendrites; and (iv) central neural pathway disorders affecting the auditory brainstem. We review data and principles to identify treatment options for affected patients and explore their benefits as a function of site of lesion. 1 Department of Audiology and Speech Pathology, The University of Melbourne, -

Correlations Between Audiogram and Objective Hearing Tests in Sensorineural Hearing Loss
International Tinnitus Journal, Vol. 5, No.2, 107-112 (1999) Correlations Between Audiogram and Objective Hearing Tests in Sensorineural Hearing Loss L. Bishara,1 J. Ben-David,l L. Podoshin,1 M. Fradis,l C.B. Teszler,l H. Pratt,2 T. Shpack,3 H. Feiglin,3 H. Hafner,3 and N. Herlinger2 I Department of Otolaryngology, Head and Neck Surgery, and 3Institute of Audiology, Bnai-Zion Medical Center, and 2Evoked Potentials Laboratory, Technion, Haifa, Israel Abstract: Owing to its subjective nature, behavioral pure-tone audiometry often is an unre liable testing method in uncooperative subjects, and assessing the true hearing threshold be comes difficult. In such cases, objective tests are used for hearing-threshold determination (i.e., auditory brainstem evoked potentials [ABEP] and frequency-specific auditory evoked potentials: slow negative response at 10 msec [SN-1O]). The purpose of this study was to evaluate the correlation between pure-tone audiogram shape and the predictive accuracy of SN-IO and ABEP in normal controls and in patients suf fering from sensorineural hearing loss (SNHL). One-hundred-and-fifty subjects aged 15 to 70, some with normal hearing and the remainder with SNHL, were tested prospectively in a double-blind design. The battery of tests included pure-tone audiometry (air and bone conduction), speech reception threshold, ABEP, and SN- 10. Patients with SNHL were divided into four categories according to audiogram shape (i.e., flat, ascending, descending, and all other shapes). The results showed that ABEP predicts behavioral thresholds at 3 kHz and 4 kHz in cases of high-frequency hearing loss. -

CASE REPORT 48-Year-Old Man
THE PATIENT CASE REPORT 48-year-old man SIGNS & SYMPTOMS – Acute hearing loss, tinnitus, and fullness in the left ear Dennerd Ovando, MD; J. Walter Kutz, MD; Weber test lateralized to the – Sergio Huerta, MD right ear Department of Surgery (Drs. Ovando and Huerta) – Positive Rinne test and and Department of normal tympanometry Otolaryngology (Dr. Kutz), UT Southwestern Medical Center, Dallas; VA North Texas Health Care System, Dallas (Dr. Huerta) Sergio.Huerta@ THE CASE UTSouthwestern.edu The authors reported no A healthy 48-year-old man presented to our otolaryngology clinic with a 2-hour history of potential conflict of interest hearing loss, tinnitus, and fullness in the left ear. He denied any vertigo, nausea, vomiting, relevant to this article. otalgia, or otorrhea. He had noticed signs of a possible upper respiratory infection, including a sore throat and headache, the day before his symptoms started. His medical history was unremarkable. He denied any history of otologic surgery, trauma, or vision problems, and he was not taking any medications. The patient was afebrile on physical examination with a heart rate of 48 beats/min and blood pressure of 117/68 mm Hg. A Weber test performed using a 512-Hz tuning fork lateral- ized to the right ear. A Rinne test showed air conduction was louder than bone conduction in the affected left ear—a normal finding. Tympanometry and otoscopic examination showed the bilateral tympanic membranes were normal. THE DIAGNOSIS Pure tone audiometry showed severe sensorineural hearing loss in the left ear and a poor speech discrimination score. The Weber test confirmed the hearing loss was sensorineu- ral and not conductive, ruling out a middle ear effusion. -

Investigations of Auditory Filters Based Excitation Patterns for Assessment of Noise Induced Hearing Loss
Investigations of Auditory Filters Based Excitation Patterns for Assessment of Noise Induced Hearing Loss Wisam Subhi Al-Dayyeni, Pengfei Sun, Jun Qin Department of Electrical and Computer Engineering, Southern Illinois University, Carbondale, IL, USA Abstract: Noise induced hearing loss (NIHL) as one of major avoidable occupational related health issues has been studied for decades. To assess NIHL, the excitation pattern (EP) has been considered as one of mechanisms to estimate movements of basilar membrane (BM) in cochlea. In this study, two auditory filters, dual resonance nonlinear (DRNL) filter and rounded- exponential (ROEX) filter, have been applied to create two EPs, referring as the velocity EP and the loudness EP, respectively. Two noise hazard metrics are also proposed based on the developed EPs to evaluate hazardous levels caused by different types of noise. Moreover, Gaussian noise and pure-tone noise have been simulated to evaluate performances of the developed EPs and noise metrics. The results show that both developed EPs can reflect the responses of BM to different types of noise. For Gaussian noise, there is a frequency shift between the velocity EP and the loudness EP. For pure-tone noise, both EPs can reflect the frequencies of input noise accurately. The results suggest that both EPs can be potentially used for assessment of NIHL. Key words: Noise induced hearing loss; excitation pattern; basilar membrane motion; auditory filter; noise assessment metrics. 1. Introduction Noise-induced hearing loss (NIHL) remains as one of the most common health related problems nowadays as stated by the World Health Organization (WHO). One of the main causes of the permanent hearing loss is the exposure to excessive noise [1-3]. -

Audiology 101: an Introduction to Audiology for Nonaudiologists Terry Foust, Aud, FAAA, CC-SLP/A; & Jeff Hoffman, MS, CCC-A
NATIONALA RESOURCE CENTER GUIDE FOR FOR EARLY HEARING HEARING ASSESSMENT DETECTION & & MANAGEMENT INTERVENTION Chapter 5 Audiology 101: An Introduction to Audiology for Nonaudiologists Terry Foust, AuD, FAAA, CC-SLP/A; & Jeff Hoffman, MS, CCC-A Parents of young Introduction What is an audiologist? children who are arents of young children who are An audiologist is a specialist in hearing identified as deaf or hard identified as deaf or hard of hearing and balance who typically works in of hearing (DHH) are P(DHH) are suddenly thrust into a either a medical, private practice, or an suddenly thrust into a world of new concepts and a bewildering educational setting. The primary roles of world of new concepts array of terms. What’s a decibel or hertz? an audiologist include the identification and a bewildering array What does sensorineural mean? Is a and assessment of hearing and balance moderate hearing loss one to be concerned problems, the habilitation or rehabilitation of terms. about, since it’s only moderate? What’s of hearing and balance problems, and the a tympanogram or a cochlear implant? prevention of hearing loss. When working These are just a few of the many questions with infants and young children, the that a parent whose child has been primary focus of audiology is hearing. identified as DHH may have. In addition to parents, questions also arise from Audiologists are licensed by the state in professionals and paraprofessionals who which they practice and may be members work in the field of early hearing detection of the American Speech-Language- and intervention (EHDI) and are not Hearing Association (ASHA), American audiologists. -
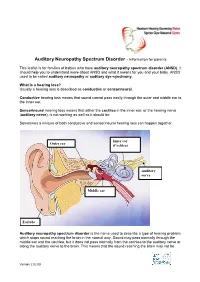
Auditory Neuropathy Spectrum Disorder - Information for Parents
Auditory Neuropathy Spectrum Disorder - Information for parents This leaflet is for families of babies who have auditory neuropathy spectrum disorder (ANSD). It should help you to understand more about ANSD and what it means for you and your baby. ANSD used to be called auditory neuropathy or auditory dys-synchrony. What is a hearing loss? Usually a hearing loss is described as conductive or sensorineural. Conductive hearing loss means that sound cannot pass easily through the outer and middle ear to the inner ear. Sensorineural hearing loss means that either the cochlea in the inner ear, or the hearing nerve (auditory nerve), is not working as well as it should be. Sometimes a mixture of both conductive and sensorineural hearing loss can happen together. Inner ear Outer ear (Cochlea) Auditory nerve Middle ear Earlobe Auditory neuropathy spectrum disorder is the name used to describe a type of hearing problem which stops sound reaching the brain in the normal way. Sound may pass normally through the middle ear and the cochlea, but it does not pass normally from the cochlea to the auditory nerve or along the auditory nerve to the brain. This means that the sound reaching the brain may not be Version 2 02.09 understood and the quality may be poor. The results of different hearing tests will help the audiologist (hearing specialist) decide if your baby has ANSD. Testing for ANSD We can carry out tests that show how well the cochlea and auditory nerve are working, even if your baby is very young. If the tests show the cochlea is working well but the auditory nerve is not working as it should be, then ANSD could be the cause. -

Review of Hair Cell Synapse Defects in Sensorineural Hearing Impairment
Otology & Neurotology 34:995Y1004 Ó 2013, Otology & Neurotology, Inc. Review of Hair Cell Synapse Defects in Sensorineural Hearing Impairment *†‡Tobias Moser, *Friederike Predoehl, and §Arnold Starr *InnerEarLab, Department of Otolaryngology, University of Go¨ttingen Medical School; ÞSensory Research Center SFB 889, þBernstein Center for Computational Neuroscience, University of Go¨ttingen, Go¨ttingen, Germany; and §Department of Neurology, University of California, Irvine, California, U.S.A. Objective: To review new insights into the pathophysiology of are similar to those accompanying auditory neuropathy, a group sensorineural hearing impairment. Specifically, we address defects of genetic and acquired disorders of spiral ganglion neurons. of the ribbon synapses between inner hair cells and spiral ganglion Genetic auditory synaptopathies include alterations of glutamate neurons that cause auditory synaptopathy. loading of synaptic vesicles, synaptic Ca2+ influx or synaptic Data Sources and Study Selection: Here, we review original vesicle turnover. Acquired synaptopathies include noise-induced publications on the genetics, animal models, and molecular hearing loss because of excitotoxic synaptic damage and subse- mechanisms of hair cell ribbon synapses and their dysfunction. quent gradual neural degeneration. Alterations of ribbon synapses Conclusion: Hair cell ribbon synapses are highly specialized to likely also contribute to age-related hearing loss. Key Words: enable indefatigable sound encoding with utmost temporal precision. GeneticsVIon -

Non-Commercial Use Only
Audiology Research 2013; volume 3:e6 Comparison of cervical and ocular vestibular evoked myogenic potentials in dancers and non-dancers Sujeet Kumar Sinha, Vaishnavi Bohra, Himanshu Kumar Sanju Department of Audiology, All India Institute of Speech and Hearing, India Abstract Introduction The objective of the study was to assess the sacculocollic and otolith In recent years, cervical vestibular evoked myogenic potentials ocular pathway function using cervical vestibular evoked myogenic (cVEMP) have been utilized for the diagnosis of various disorders such potentials (cVEMP) and ocular vestibular myogenic potentials as, Meniere’s disease,1,2 acoustic neuroma,2-5 superior canal dehis- (oVEMP) in dancers and non dancers. Total 16 subjects participated in cence,6 vestibular neuritis,7 benign paroxysmal positional vertigo,8 the study. Out of 16 participants, 8 were trained in Indian classical noise induced hearing loss,9,10 auditory neuropathy/audiovestibular form of dance (dancers) and other 8 participants who were not trained neuropathy,10,11 as well as other disorders such as cerebellopontine in any dance form (non dancers). cVEMP and oVEMP responses were angle tumor,12 and multiple sclerosis.2 Similarly, ocular vestibular recorded for all the subjects. Non Parametric Mann-Whitney U test evoked myogenic potentials (oVEMP) also have been utilised in diag- revealed no significant difference between dancers and non dancers 13 for the latency and amplitude parameter for cVEMP and oVEMP, i.e. nosing superior semicircular canal dehiscence syndrome, internu- 14 P13, N23 latency and P13-N23 complex amplitude and N10, P14 laten- clearophthalmoplegia, to differentiateonly between cerebellar and brain- cy, N10-P14 complex amplitude respectively. -

MEDICINE TODAY Audiometry -~60
9 November 196S Schizophrenia-Freeman MEDIALSHRNAL 373 In the Salford comprehensive community mental health ser- FURTHER READING vice vulnerable cases of schizophrenia have been treated with Bennett, D. H., New Aspects of the Mental Health Services, ed. H. L. Frceman and J. Farndale, 1967. Oxford. this preparation for nearly two years and experience has been Brown, G. W., Bone, M., Dalison, B., and Wing, J. K., Schizophrenia gained in over 100 cases. This confirms results from elsewhere and Social Care, 1966. London. Br Med J: first published as 10.1136/bmj.4.5627.373 on 9 November 1968. Downloaded from Kinross-Wright, J., and Charalampous, K. D., Int. 7. Neuropsychiat., that it represents an important step forward in the community 1965, 1, 66. management of schizophrenia. The injections may be given in Psychiatric Hospital Care, ed. H. L. Freeman, 1965. London. Treatment of Mental Disorders in the Community, ed. G. R. Daniel and hospital clinics, at general practitioners' surgeries, or by nurses 1968. London. at the patients' homes. An interested family doctor can H. L. Freeman, certainly make a big contribution to the community care of his schizophrenic patients by undertaking these injections, since it is possible to do a rapid check on the mental state B.M.J. Publications at the same time, or perhaps receive a report from an The following are available from the Publishing Manager, B.M.A. accompanying relative. It may also be necessary to issue House, Tavistock Square, London W.C.1. The prices include regular prescriptions for antiparkinsonian drugs, since side- postage. effects are fairly common, at least in the early stages of the The New Gcneral Practice .. -

Cranial Nerve VIII
Cranial Nerve VIII Color Code Important (The Vestibulo-Cochlear Nerve) Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, the students should be able to: ✓ List the nuclei related to vestibular and cochlear nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the vestibular pathways and its main connections. ✓ Describe the auditory pathway and its main connections. Due to the difference of arrangement of the lecture between the girls and boys slides we will stick to the girls slides then summarize the pathway according to the boys slides. Ponto-medullary Sulcus (cerebello- pontine angle) Recall: both cranial nerves 8 and 7 emerge from the ventral surface of the brainstem at the ponto- medullary sulcus (cerebello-pontine angle) Brain – Ventral Surface Vestibulo-Cochlear (VIII) 8th Cranial Nerve o Type: Special sensory (SSA) o Conveys impulses from inner ear to nervous system. o Components: • Vestibular part: conveys impulses associated with body posture ,balance and coordination of head & eye movements. • Cochlear part: conveys impulses associated with hearing. o Vestibular & cochlear parts leave the ventral surface* of brain stem through the pontomedullary sulcus ‘at cerebellopontine angle*’ (lateral to facial nerve), run laterally in posterior cranial fossa and enter the internal acoustic meatus along with 7th (facial) nerve. *see the previous slide Auditory Pathway Only on the girls’ slides 04:14 Characteristics: o It is a multisynaptic pathway o There are several locations between medulla and the thalamus where axons may synapse and not all the fibers behave in the same manner. -

Audiology and Hearing Aid Services
For more information, call the Hearing Aid Services office nearest you: Comprehensive hearing aid related services Barbourville Bowling Green are available to children diagnosed with (800) 348-4279 (800) 843-5877 permanent childhood hearing loss (PCHL). (606) 546-5109 (270) 746-7816 Elizabethtown Hazard Who should be referred to the OCSHCN (800) 995-6982 (800) 378-3357 Hearing Aid Services program? (270) 766-5370 (606) 435-6167 Children who are in need of new or Lexington Louisville replacement hearing aids and want to (800) 817-3874 (800) 232-1160 receive hearing aids and related services (859) 252-3170 (502) 429-4430 through a OCSHCN audiologist and wish to Morehead Owensboro receive Otology care outside of the (800) 928-3049 (877) 687-7038 clinical Otology program. (606) 783-8610 (270) 687-7038 What audiology services are available Paducah Prestonsburg (800) 443-3651 (800) 594-7058 through the OCSHCN Hearing Aid Services (270) 443-3651 (606) 889-1761 program? Licensed, certified audiologists conduct Somerset (800) 525-4279 periodic comprehensive hearing evaluations, (606) 677-4120 hearing aid checks, hearing aid repairs and Audiology and hearing aid evaluations according to ASHA best practices guidelines. Comprehensive Kentucky Cabinet for Health and Family Services Hearing Aid Services Office for Children with Special Health Care Needs reports are provided to the managing 310 Whittington Parkway, Suite 200, Louisville, KY 40222 otolaryngologist on an on-going basis; Phone: (502) 429-4430 or (800) 232-1160 FAX: (502) 429-4489 additional follow up testing will be http://chfs.ky.gov/agencies/ccshcn Information for Parents and Equal Opportunity Employer M/D/F completed at physician request. -

The Genetic Relationship Between Paroxysmal Movement Disorders and Epilepsy
Review article pISSN 2635-909X • eISSN 2635-9103 Ann Child Neurol 2020;28(3):76-87 https://doi.org/10.26815/acn.2020.00073 The Genetic Relationship between Paroxysmal Movement Disorders and Epilepsy Hyunji Ahn, MD, Tae-Sung Ko, MD Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Korea Received: May 1, 2020 Revised: May 12, 2020 Seizures and movement disorders both involve abnormal movements and are often difficult to Accepted: May 24, 2020 distinguish due to their overlapping phenomenology and possible etiological commonalities. Par- oxysmal movement disorders, which include three paroxysmal dyskinesia syndromes (paroxysmal Corresponding author: kinesigenic dyskinesia, paroxysmal non-kinesigenic dyskinesia, paroxysmal exercise-induced dys- Tae-Sung Ko, MD kinesia), hemiplegic migraine, and episodic ataxia, are important examples of conditions where Department of Pediatrics, Asan movement disorders and seizures overlap. Recently, many articles describing genes associated Medical Center Children’s Hospital, University of Ulsan College of with paroxysmal movement disorders and epilepsy have been published, providing much infor- Medicine, 88 Olympic-ro 43-gil, mation about their molecular pathology. In this review, we summarize the main genetic disorders Songpa-gu, Seoul 05505, Korea that results in co-occurrence of epilepsy and paroxysmal movement disorders, with a presenta- Tel: +82-2-3010-3390 tion of their genetic characteristics, suspected pathogenic mechanisms, and detailed descriptions Fax: +82-2-473-3725 of paroxysmal movement disorders and seizure types. E-mail: [email protected] Keywords: Dyskinesias; Movement disorders; Seizures; Epilepsy Introduction ies, and paroxysmal dyskinesias [3,4]. Paroxysmal dyskinesias are an important disease paradigm asso- Movement disorders often arise from the basal ganglia nuclei or ciated with overlapping movement disorders and seizures [5].