Cephalometric Measures Correlate with Polysomnography Parameters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Investigating the Various Shapes of Sella Turcica in Nigerian Children Using Lateral Skull Radiographs
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Investigating the Various Shapes of Sella Turcica in Nigerian Children Using Lateral Skull Radiographs Bello A., Usman J.D. Department of Anatomy, Faculty of Basic Medical Sciences, College of Health Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria. Corresponding Author: Bello A ABSTRACT The knowledge of the normal radiographic anatomy of the sella turcica and sella point is of great importance to clinicians in enabling them quickly recognize, investigate or evaluate any deviation from normal as well as any pathological situation related to the pituitary gland. This study investigated the various shapes of the sella turcica in children. A total of 250 lateral skull radiographs taken in the Department of Radiology, Usmanu Danfodiyo University Teaching Hospital (UDUTH), Sokoto from January 2013 to December 2014 were retrieved for the purpose of this study. Radiographs were mounted on the viewing box and variants of the anatomical shapes of the sella turcica were studied and classified. Of the 162 radiographs used in this study, 114 (70.4%) sella turcica were round shaped while 48 (29.6%) oval in shaped. This observed difference was statistically significant (p<0.001). Meanwhile, the floor of the sella turcica of the study participants showed a concave outline in 130 (80%) of the children and flat outline in 32 (20%) of the children. Sexual dimorphism was seen in this study with respect to shape of sella turcica. Round shaped sella turcica was predominant in Nigerian children used in this study. The prevalence and the relative frequencies of the normal variants of the anatomical shapes of the sella turcica of male Nigerian children differ significantly from those of their female counterparts. -

MBB: Head & Neck Anatomy
MBB: Head & Neck Anatomy Skull Osteology • This is a comprehensive guide of all the skull features you must know by the practical exam. • Many of these structures will be presented multiple times during upcoming labs. • This PowerPoint Handout is the resource you will use during lab when you have access to skulls. Mind, Brain & Behavior 2021 Osteology of the Skull Slide Title Slide Number Slide Title Slide Number Ethmoid Slide 3 Paranasal Sinuses Slide 19 Vomer, Nasal Bone, and Inferior Turbinate (Concha) Slide4 Paranasal Sinus Imaging Slide 20 Lacrimal and Palatine Bones Slide 5 Paranasal Sinus Imaging (Sagittal Section) Slide 21 Zygomatic Bone Slide 6 Skull Sutures Slide 22 Frontal Bone Slide 7 Foramen RevieW Slide 23 Mandible Slide 8 Skull Subdivisions Slide 24 Maxilla Slide 9 Sphenoid Bone Slide 10 Skull Subdivisions: Viscerocranium Slide 25 Temporal Bone Slide 11 Skull Subdivisions: Neurocranium Slide 26 Temporal Bone (Continued) Slide 12 Cranial Base: Cranial Fossae Slide 27 Temporal Bone (Middle Ear Cavity and Facial Canal) Slide 13 Skull Development: Intramembranous vs Endochondral Slide 28 Occipital Bone Slide 14 Ossification Structures/Spaces Formed by More Than One Bone Slide 15 Intramembranous Ossification: Fontanelles Slide 29 Structures/Apertures Formed by More Than One Bone Slide 16 Intramembranous Ossification: Craniosynostosis Slide 30 Nasal Septum Slide 17 Endochondral Ossification Slide 31 Infratemporal Fossa & Pterygopalatine Fossa Slide 18 Achondroplasia and Skull Growth Slide 32 Ethmoid • Cribriform plate/foramina -

Sella Turcica Morphology on Cephalometric Radiographs and Dental Abnormalities—Is There Any Association? —Systematic Review
International Journal of Environmental Research and Public Health Review Sella Turcica Morphology on Cephalometric Radiographs and Dental Abnormalities—Is There Any Association? —Systematic Review Tomasz Jankowski 1, Maciej Jedli ´nski 2 , Katarzyna Grocholewicz 2 and Joanna Janiszewska-Olszowska 2,* 1 Private Practice Dental Clinic Jankowscy, 68-200 Zary,˙ Poland; [email protected] 2 Department of Interdisciplinary Dentistry, Pomeranian Medical University in Szczecin, 70-204 Szczecin, Poland; [email protected] (M.J.); [email protected] (K.G.) * Correspondence: [email protected]; Tel.: +48-91-466-1690 Abstract: Background: The sella turcica is a saddle-like structure in the middle cranial fossa on the intracranial surface of the sphenoid bone, visible on lateral cephalograms routinely conducted for orthodontic diagnosis. The development of facial structures follows similar traits to the sella turcica: glandular anomalies may be associated with functional disorders, e.g., altered hormonal levels, thus influencing dental development. The aim of this study is to find out if there is any association between the morphology of the sella turcica on cephalometric radiographs and the presence of dental abnormalities. (2) Methods: The search was conducted on 27 January 2021 in four search engines: Medline (PubMed Central), Scopus, Web of Science, Embase. The keywords used in the search strategy were as follows: “sella turcica” AND (“dental abnormalities” OR “dental anomalies” OR Citation: Jankowski, T.; Jedli´nski,M.; “malocclusion”). Since all the studies finally included were retrospective case–control studies, the Grocholewicz, K.; Janiszewska- Newcastle–Ottawa Quality Assessment Form for Case–Control Studies was applied. (3) Results: The Olszowska, J. Sella Turcica search strategy identified 465 articles: 289 from PubMed, 121 from Scopus, 32 from Web of Science and Morphology on Cephalometric 23 from Embase. -

Level I to III Craniofacial Approaches Based on Barrow Classification For
Neurosurg Focus 30 (5):E5, 2011 Level I to III craniofacial approaches based on Barrow classification for treatment of skull base meningiomas: surgical technique, microsurgical anatomy, and case illustrations EMEL AVCı, M.D.,1 ERINÇ AKTÜRE, M.D.,1 HAKAN SEÇKIN, M.D., PH.D.,1 KUTLUAY ULUÇ, M.D.,1 ANDREW M. BAUER, M.D.,1 YUSUF IZCI, M.D.,1 JACQUes J. MORCOS, M.D.,2 AND MUSTAFA K. BAşKAYA, M.D.1 1Department of Neurological Surgery, University of Wisconsin–Madison, Wisconsin; and 2Department of Neurological Surgery, University of Miami, Florida Object. Although craniofacial approaches to the midline skull base have been defined and surgical results have been published, clear descriptions of these complex approaches in a step-wise manner are lacking. The objective of this study is to demonstrate the surgical technique of craniofacial approaches based on Barrow classification (Levels I–III) and to study the microsurgical anatomy pertinent to these complex craniofacial approaches. Methods. Ten adult cadaveric heads perfused with colored silicone and 24 dry human skulls were used to study the microsurgical anatomy and to demonstrate craniofacial approaches in a step-wise manner. In addition to cadaveric studies, case illustrations of anterior skull base meningiomas were presented to demonstrate the clinical application of the first 3 (Levels I–III) approaches. Results. Cadaveric head dissection was performed in 10 heads using craniofacial approaches. Ethmoid and sphe- noid sinuses, cribriform plate, orbit, planum sphenoidale, clivus, sellar, and parasellar regions were shown at Levels I, II, and III. In 24 human dry skulls (48 sides), a supraorbital notch (85.4%) was observed more frequently than the supraorbital foramen (14.6%). -

Effects of Morphological Changes in Sella Turcica: a Review
European Journal of Molecular & Clinical Medicine ISSN 2515-8260 Volume 07, Issue 03, 2020 1662 EFFECTS OF MORPHOLOGICAL CHANGES IN SELLA TURCICA: A REVIEW Chandrakala B1, Govindarajan Sumathy2, Bhaskaran Sathyapriya*, Pavishwarya P3, Sweta Jain3 1. Senior Lecturer, Department of Anatomy, Sree Balaji Dental College & Hospital, Bharath Institute of Higher Education & Research, Chennai. 2. Professor and Head, Department of Anatomy, Sree Balaji Dental College & Hospital, Bharath Institute of Higher Education & Research, Chennai. 3. Graduate student, Sree Balaji Dental College and Hospital, Bharath Institute of Higher Education and Research *Professor, Department of Anatomy, Sree Balaji Dental College & Hospital, Bharath Institute of Higher Education & Research, Chennai. Corresponding author: Dr. Bhaskaran Sathyapriya Professor, Department of Anatomy, Sree Balaji Dental College & Hospital, Bharath Institute of Higher Education & Research, Chennai. ABSTRACT Sella turcica is a saddle shaped bony structure present on the sphenoid bone. The pituitary gland is seated at the inferior aspect of the sella turcica, called hypophyseal fossa. Sella turcica serves as a cephalometric landmark, that being said any morphological changes can affect the overall craniometry of the individual as well as alter the function of the structures it lodges. The following review emphasis on the possible morphological changes of sella turcica and its effects on the individual. Keywords: Pituitary gland, morphology, bridge, foramen, bone. 1662 European Journal of Molecular -
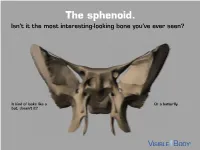
The Sphenoid. Isn’T It the Most Interesting-Looking Bone You’Ve Ever Seen?
The sphenoid. Isn’t it the most interesting-looking bone you’ve ever seen? It kind of looks like a Or a butterfly. bat, doesn’t it? The sphenoid articulates with 12 bones, both in the neurocranium and facial skeleton. The sphenoid is not just present in human skeletons, but also in mammalian ones. : A human has 1 Factoid sphenoid bone, but a dog has 8 bones that make up its sphenoid. The sphenoid is one of the 8 bones of the neurocranium (bones that protect the brain). It is the keystone* bone at the base of the skull. *In architecture, a keystone is the piece at the apex of an arch, locking all the other pieces together and bearing the weight of it all. The body of the sphenoid is the central part of the bone. It is a hollowed-out, cubical portion of the bone that forms the sphenoidal sinuses. The body is home to a deep depression known as the Sella turcica, which houses the pituitary gland. Factoid: sella turcica is Latin for “Turkish saddle” because of its resemblance to the saddles used by Turks, which had supports in the front and back. The greater wings of the sphenoid articulate with several bones, including the frontal, temporal, parietal, and zygomatic. They also serve as the attachment site for the temporalis muscles. The lesser wings are thin, triangular plates located above the greater wings. They, along with the body, form the optic canal. The optic nerve (II) passes through the optic canal to the eyes. The lateral and medial pterygoid plates project downward from the sphenoid body to give shape to the nasal cavity. -

Elsberg, Hare, and Duke, 1932)
Br J Ophthalmol: first published as 10.1136/bjo.51.12.829 on 1 December 1967. Downloaded from Brit. J. Ophthal. (1967) 51, 829 MISINTERPRETATION OF SPHENOIDAL RIDGE MEN1NGIOMATA*t BY ALY MORTADA Department of Ophthalmology, Faculty ofMedicine, Cairo University, Cairo, Egypt SPHENOIDAL ridge meningiomata are a common cause ofunilateral proptosis (Elsberg, Hare, and Duke, 1932). Ophthalmologists are more familiar with the classical picture of sphenoidal ridge meningioma usually occurring in adults and giving rise to unilateral proptosis (Fig. 1), optic atrophy, oculomotor palsies, anaesthesia in the distribution ofthe fifth nerve, and (rarely) pituitary disturbance and uncinate fits. 1.-Right gradual progressive proptosis of 4 years' duration due to a right sphenoidal ridge meningioma in a woman aged 40 years. copyright. .. .. .. [...._ R...........FIG. The characteristic x ray reveals involvement of the lesser and greater wings of the sphenoid bone by dense hyperostosis, which is usually diffuse (Figs 2 and 3) diminish- ing the-:, size of the orbit, optic canal, and superior orbital fissure. :;......... http://bjo.bmj.com/ on October 2, 2021 by guest. Protected _~~~~~~~~~~~~~~~~~~~~X. FIG. 2.-Postero-anterior x ray, showing typical diffuse hyperostosis of right lesser FIG. 3.-Lateral x ray, showing diffuse hyperostosis of and greater wings of sphenoid bone due to lesser and greater wings extending to body of sphenoid sphenoidal ridge meningioma. bone, narrowing the sphenoidal sinus but not affecting the sella turcica, due to right sphenoidal ridge menin- gioma. Swelling of the temple may also occur (Figs 4 and 5, overleaf). * Received for publication October 3, 1966. t Address for reprints: 18A 26th July St., Cairo, Egypt. -

Trans-Foramen Magnum Examination of the Sella Turcica Region in the Macerated Human Skulls
FOLIA MEDICA CRACOVIENSIA 25 Vol. LIII, 3, 2013: 25–32 PL ISSN 0015-5616 JANUSZ SKRZAT1, MAGDALENA KOZERSKA1, Przemysław Chmielewski1, GrzeGorz GonCerz1 TRANS-FORAMEN MAGNUM EXAMINATION OF THE SELLA TURCICA REGION IN THE MACERATED HUMAN SKULLS Abstract: This report presents a method of quick and accurate imaging of the sellar region by means of the laryngological mirror equipped with a light pipe and followed by taking digital photograph as the mirror image visible through the foramen magnum. A technique of the intracranial imaging of the osseous structures was tested on the macerated human skulls. Images of the sellar region were presented as the example of quality of the employed technique, which can be regarded as a simplified version of the endoscopic examination. Key words: visual inspection, digital imaging, sella turcica. INTRODUCTION The morphology and variability of the sellar region have been of interest of both of anatomists and clinicians. A particular attention was paid to heterotopic ossi- fication and calcification which occur sometimes in the bands of the dura mater attached to the sphenoid bone [1]. An abnormal appearance of the sella turcica region was usually detected and examined by the radiographic techniques (X-ray pictures or CT-scans) [2, 3]. Also, an anatomical study of the sellar region was performed on dry human skulls after removing the calvaria to reveal interior of the skull [4]. Although computed tomography is in widespread use for clinical imaging, a simple imaging equipment combined with the digital photography can provide satisfactory results for anatomical studies. In some circumstances, the normal or abnormal morphology of the intracranial structures can be presented directly by the digital photography. -

Sphenoid Sinus Mucoceles
1. Neurosurg. / Volume 32 / April, 1970 Sphenoid Sinus Mucoceles G. ROBERT NUGENT, M.D., PHILIP SPRINKLE, M.D., AND BYRON M. BLOOR, M.D. Division o/Neurological Surgery, and Division oJ Otolaryngological Surgery, West Virginia University Medical Center, Morgantown, West Virginia HE neurosurgeon is familiar with the The etiology of a mucocele is conjectural. frontal sinus mucocele as a common It would be convenient to consider them as T cause of unilateral exophthalmos and simple retention cysts of the sinus resulting disorders of eye movement, but the less from inflammatory blockage of the draining common sphenoid sinus mucocele may have ostium. However, they do occur when the escaped his experience. These lesions are po- ostium is patent. Nor does a blocked ostium tentially more serious, and are often misdi- always result in mucoceles. 17,47,61 Others agnosed and operated on as pituitary tu- have considered them as originating from mors. A mucocele of the sphenoid sinus was cystic dilatation of the mucus glands of the first described in 1889 by Berg? Since then epithelial lining of the sinus, or from the there have been sporadic reports, primarily cystic degeneration of contained polyps. 3,17 in the European literature, the best being SchiillerG4 has raised the possibility of the that of Lundgren and Olin. 4~ It is the pur- development of a hypophyseal cyst from cell pose of this paper to review the world litera- rests in the inner or under half of the sella ture, add two additional cases, and alert the turcica, but evidence for this is lacking. -

Bridges of the Sella Turcica — Anatomy and Topography
FOLIA MEDICA CRACOVIENSIA 97 Vol. LII, 3–4, 2012: 97–101 PL ISSN 0015-5616 JANUSZ SKRZAT1, Izabela Mróz1, JUSTYNA MARCHEWKA1,2 BRIDGES OF THE SELLA TURCICA — ANATOMY AND TOPOGRAPHY Abstract: Bridges of the sella turcica — anatomy and topography This paper presents anatomy and topography of the inconstant osseous bridges that may occur in the sella turcica region. The interclinoid bridge and the caroticoclinoid bridge can be formed in con- sequence of abnormal ossification of the dural folds or disturbances in development of the sphenoid bone. Their presence may be of clinical importance because of potential influence on the neurovascular structures passing in the vicinity of the clinoid processes of the sphenoid bone. Key words: sellar bridge, sella turcica, sphenoid bone INTRODUCTION Process of ossification of cranial structures might be a natural consequence of ageing or a result of adaptation changes of the axial skeleton, although sometimes it is difficult to guess what are the real causative factors [1–3]. The folds of the dura mater (ligaments) that are attached to the clinoid processes (the anterior, the middle and the posterior) may occasionally ossify and form bony bridges of the sphenoid bone. These inconstant osseous structures may also derive from the cartilaginous tissue [4, 5]. The formation of the osseous bridges within the sellar region may also effect of disturbances in development of the sphenoid bone [4, 6]. The ligament between anterior and posterior clinoid process is known as the interclinoid ligament, and the bony connection between these processes is known as the interclinoid bridge. In turn, the anterior and middle clinoid processes may be connected by the caroticoclinoid ligament which may ossify forming a caroticoclinoid bridge. -

Skull. Sphenoid and Ethmoid Bones
Skull. Sphenoid and Ethmoid bones PhD., Dr. David Lendvai Department of Anatomy, Histology and Embriology Semmelweis University, Faculty of Medicine 2018. Skeletal system Structure of the skull Border between viscerocrainum and neurocranium Calvaria Main parts of the skull •Constitute by 22 bones: •neurocranium (8) – UNPAIRED: frontal, occipital, sphenoid, ethmoid bones PAIRED: temporal, parietal bones •viscerocranium (14) -UNPAIRED: mandibule, vomer. PAIRED: nasal, maxilla, zygomatic, lacrimal, palatine, inferior nasal concha Their role – formation of cavities, protect viscera, voice formation, initial portions of the gastrointerstinal and respiratory systems, insertion of muscles (mascication, head movements) Cavities: - Cranial cavity, - Nasal cavity, - Paranasal sinuses - Oral cavity, - Orbit, - (Tympanic cavity, Inner ear) Connections between cranial bones • Synchondrosis, synostosis (cartilagineal and bony connections) • Sutures – Coronal – Sagittal – Lambdoid Calvaria and the base of the skull Calvaria External aspect of the calvaria Base of the skull Internal aspect of the calvaria Fossae cranii Anterior cranial fossa MIddle cranial fossa Posterior cranial fossa • Posterior cranial fossa: • Anterior cranial fossa: • Occipital, temporal bones, frontal, ethmoid, lesser wings parietal bones of sphenoid • Middle cranial fossa: sphenoid, temporal bones, parietal bones Bones of the neurocranium Parietal bone Frontal bone Temporal bone Ethmoid Sphenoid Sphenoid bone Braus Part of the external skull base (- body (Corpus), pterygoid process, -

3 the Sphenoid Bone More
I have a video that takes you #3 The Sphenoid bone through this! E CLICK HERE This is one of the hardest cranial bones. It is a single bone that makes up the Read If you are having a Me majority of the base of the skull, just anterior to the occipital. hard time seeing There are outside features, which can be seen on the base, and inside features, the sphenoid as a which can be seen on the floor. The floor features are associated with the single bone, see brain. The base features are mostly large muscle attachments that let you the next page move your skull and jaw. Inferior view: Pterygoid processes or plates Muscle attachments. “Ptery” means winged, like “pterodactyl”. Superior view: Greater Wing of the Sphenoid The large fossa Lesser Wing of the Sphenoid Sella The smaller upper “ridge” turcica = If you are having a Turkish hard time seeing Sella Turcica, with it’s parts: saddle the sella turcica Means “Turkish saddle” in Latin. See and its parts, I image. have a “trick” on the next page! Hypophyseal Fossa “Seat” of the saddle. The pituitary gland sits here. Anterior Clinoid Process Muscle attachments. Look like the “front handles” on the saddle’ Optic Foramina (runs right next to anterior clinoid processes) Not shown in image. Run forward, to the eyes, just next to the anterior clinoid processes. more There are also a lot of foramen, but we’ll deal with those in their own module later! This page contains some help with 2 difficult concepts. You can skip it if you do not need it Having a hard time seeing the sphenoid as a single bone? After all, it looks like it is paired, forming part of the eye orbit: But this is reality: its wings wraps around up into the orbits.