Mini-Symposium-NCCS-Oct17.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CDRI Awards 2021 for Excellence in Drug Research
CDRI AWARDS for Excellence in Drug Research 2021 Invitation for Nominations / Applications Instituted by the CSIR-Central Drug Research Institute, Lucknow – 226 031 CDRI AWARDS for Excellence in Drug Research Preamble The CSIR-Central Drug Research Institute (CDRI), Lucknow, was seventh in the chain of CSIR labs that were established in India right after independence India with an aim for technological independence of the Nation. CSIR-CDRI was broadly mandated with the task to revolutionise the pharmaceutical sector, which was almost non-existent at that time for accessibility and affordability of drugs. Today, CSIR-CDRI has evolved into a unique institution possessing end-to-end expertise in the domain of new drug development –its human resource and infrastructure today is second to none. Besides being the breeding ground of huge highly trained human resource for the drug development and manufacturing, out of 20 new drugs discovered, developed and approved in the post-independent India, 11 (8 synthetic + 3 phytopharmaceuticals) are contributions of CSIR-CDRI, Lucknow. These include Centchroman, Arteether, Centbucridine, Gugulipid, Bacosides Enriched Standardized Extract of Bacopa, etc. Centchroman (Ormeloxifene), a non-steroidal contraceptive with almost no toxicity, is now part of the National Family Program, whereas α-β Arteether (antimalarial) is included in the National Malaria Program. However, from the National front, new drug discovery and development is still in its infancy in India. India is a leader in global generic pharmaceuticals manufacturing and is being called as Pharmacy of the developing world, however, many generics manufactured in India are at the end of their respective product life cycles. -

Insights from a Pan India Sero- Epidemiological Survey
SHORT REPORT Insights from a Pan India Sero- Epidemiological survey (Phenome-India Cohort) for SARS-CoV2 Salwa Naushin1,2†, Viren Sardana1,2†, Rajat Ujjainiya1,2, Nitin Bhatheja1, Rintu Kutum1,2, Akash Kumar Bhaskar1,2, Shalini Pradhan1, Satyartha Prakash1, Raju Khan2,3, Birendra Singh Rawat2,4, Karthik Bharadwaj Tallapaka5, Mahesh Anumalla5, Giriraj Ratan Chandak2,5, Amit Lahiri2,6, Susanta Kar2,6, Shrikant Ramesh Mulay2,6, Madhav Nilakanth Mugale2,6, Mrigank Srivastava2,6, Shaziya Khan2,6, Anjali Srivastava2,6, Bhawana Tomar2,6, Murugan Veerapandian2,7, Ganesh Venkatachalam2,7, Selvamani Raja Vijayakumar7, Ajay Agarwal2,8, Dinesh Gupta8, Prakash M Halami2,9, Muthukumar Serva Peddha2,9, Gopinath M Sundaram2,9, Ravindra P Veeranna2,9, Anirban Pal2,10, Vinay Kumar Agarwal10, Anil Ku Maurya10, Ranvijay Kumar Singh2,11, Ashok Kumar Raman11, Suresh Kumar Anandasadagopan2,12, Parimala Karuppanan12, Subramanian Venkatesan2,12, Harish Kumar Sardana13, Anamika Kothari13, Rishabh Jain2,13, Anupama Thakur2,13, Devendra Singh Parihar2,13, Anas Saifi2,13, Jasleen Kaur2,13, Virendra Kumar13, Avinash Mishra2,14, Iranna Gogeri2,15, Geethavani Rayasam2,16, Praveen Singh1,2, Rahul Chakraborty1,2, Gaura Chaturvedi1,2, Pinreddy Karunakar1,2, Rohit Yadav1,2, Sunanda Singhmar1, Dayanidhi Singh1,2, Sharmistha Sarkar1,2, Purbasha Bhattacharya1,2, Sundaram Acharya1,2, Vandana Singh1,2, Shweta Verma1,2, Drishti Soni1,2, Surabhi Seth1,2, Sakshi Vashisht1,2, Sarita Thakran1,2, Firdaus Fatima1,2, Akash Pratap Singh1,2, Akanksha Sharma1,2, Babita Sharma1,2, *For -

Adipose Recruitment and Activation of Plasmacytoid Dendritic Cells Fuel Metaflammation’
Diabetes Page 2 of 61 Adipose recruitment and activation of plasmacytoid dendritic cells fuel metaflammation Amrit Raj Ghosh1, Roopkatha Bhattacharya1, Shamik Bhattacharya1, Titli Nargis2, Oindrila Rahaman1, Pritam Duttagupta1, Deblina Raychaudhuri1, Chinky Shiu Chen Liu1, Shounak Roy1, Parasar Ghosh3, Shashi Khanna4, Tamonas Chaudhuri4, Om Tantia4, Stefan Haak5, Santu Bandyopadhyay1, Satinath Mukhopadhyay6, Partha Chakrabarti2 and Dipyaman Ganguly1*. Divisions of 1Cancer Biology & Inflammatory Disorders and 2Cell Biology & Physiology, CSIR- Indian Institute of Chemical Biology, Kolkata, India; 4ILS Hospitals, Kolkata, India; 5Zentrum Allergie & Umwelt (ZAUM), Technical University of Munich and Helmholtz Centre Munich, Munich, Germany; Departments of 3Rheumatology and 6Endocrinology, Institute of Postgraduate Medical Education & Research, Kolkata, India. *Corresponding author: Dipyaman Ganguly, Division of Cancer Biology & Inflammatory Disorders, CSIR-Indian Institute of Chemical Biology, 4 Raja S C Mullick Road, Jadavpur, Kolkata, West Bengal, India, 700032. Phone: 91 33 24730492 Fax: 91 33 2473 5197 Email: [email protected] Running title: PDCs and type I interferons in metaflammation Word count (Main text): 5521 Figures: 7, Table: 1 1 Diabetes Publish Ahead of Print, published online August 25, 2016 Page 3 of 61 Diabetes ABSTRACT In obese individuals the visceral adipose tissue (VAT) becomes seat of chronic low grade inflammation (metaflammation). But the mechanistic link between increased adiposity and metaflammation remains largely -

National Institutional Ranking Framework
National Institutional Ranking Framework Ministry of Human Resource Development Government of India Welcome to Data Capturing System: OVERALL Submitted Institute Data for NIRF'2020' Institute Name: ACADEMY OF SCIENTIFIC & INNOVATIVE RESEARCH [IR-O-U-0713] Sanctioned (Approved) Intake Academic Year 2018-19 2017-18 2016-17 2015-16 2014-15 2013-14 PG [1 Year Program(s)] 17 - - - - - PG [2 Year Program(s)] 136 65 - - - - Total Actual Student Strength (Program(s) Offered by Your Institution) (All programs No. of Male No. of Female Total Students Within State Outside State Outside Economically Socially No. of students No. of students No. of students No. of students of all years) Students Students (Including male (Including male Country Backward Challenged receiving full receiving full receiving full who are not & female) & female) (Including male (Including male (SC+ST+OBC tuition fee tuition fee tuition fee receiving full & female) & female) Including male reimbursement reimbursement reimbursement tuition fee & female) from the State from Institution from the Private reimbursement and Central Funds Bodies Government PG [1 Year 6 3 9 2 7 0 0 2 0 0 0 2 Program(s)] PG [2 Year 53 56 109 48 61 0 1 42 1 1 0 41 Program(s)] Placement & Higher Studies PG [1 Years Program(s)]: Placement & higher studies for previous 3 years Academic Year No. of first year No. of first year Academic Year No. of students graduating in minimum No. of students Median salary of No. of students students intake in the students admitted in stipulated time placed placed selected for Higher year the year graduates(Amount in Studies Rs.) 2016-17 20 20 2016-17 17 5 319000(Rupees Three 9 Lakhs Nineteen Thousand Only) 2017-18 16 16 2017-18 16 10 330000(Rupees Three 2 Lakhs Thirty Thousand Only) 2018-19 17 9 2018-19 9 2 325000(Rupees Three 4 Lakhs Twenty Five Thousand Only) PG [2 Years Program(s)]: Placement & higher studies for previous 3 years Academic Year No. -

Office of the Public Relations Officer- Media Coordinator Jamia Millia Islamia
Office of the Public Relations Officer- Media Coordinator Jamia Millia Islamia December 26, 2018 Press Release Jamia Professor honored with National Bioscience Award by Department of Biotechnology, Govt of India Prof. Mohammad Zahid Ashraf has been honoured with National Bio-science Award 2018 as recognition of his research excellence. This award instituted by the Department of Biotechnology of the Government of India recognizes research and development work carried out in India during the last 5 years of the career. The award is given to Indian bio-scientists of less than 45 years of age, who has made unique contributions towards the development of state of art in basic and applied areas of biological sciences. The award carries a citation, a plaque, a cash prize of Rs 300,000 and a research support grant of Rs 15,00,000 for three years. It is considered as one of the highest Indian science awards and as equivalent to the Shanti Swarup Bhatnagar Prize given by the Council of Scientific and Industrial Research of India. Dr. Ashraf is currently a Professor in the Department of Biotechnology at Faculty of Natural Sciences, Jamia Millia Islamia (JMI), New Delhi. Prior to this he was Head of the Genomics Division at Defence Institute of Physiology and Allied Sciences (DIPAS), Defence Research and Development Organization (DRDO), Delhi. Dr. Ashraf is pioneer in the field of high altitude thrombosis and has done novel work in resolving the mystery of blood clotting on exposure to hypoxia at high altitudes in Soldiers posted at extreme altitudes including Siachen Glacier. His research has unraveled many problems associated with high altitude thrombosis and mechanisms associated with its pathology. -

The Role of Dendritic Cells in Autoimmunity Dipyaman Ganguly, Stefan Haak, Vanja Sisirak, Boris Reizis
The role of dendritic cells in autoimmunity Dipyaman Ganguly, Stefan Haak, Vanja Sisirak, Boris Reizis To cite this version: Dipyaman Ganguly, Stefan Haak, Vanja Sisirak, Boris Reizis. The role of dendritic cells in au- toimmunity. Nature Reviews Immunology, Nature Publishing Group, 2014, 13, pp.566 - 577. 10.1038/nri3477. hal-03045723 HAL Id: hal-03045723 https://hal.archives-ouvertes.fr/hal-03045723 Submitted on 8 Dec 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. NIH Public Access Author Manuscript Nat Rev Immunol. Author manuscript; available in PMC 2014 September 11. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Nat Rev Immunol. 2013 August ; 13(8): 566–577. doi:10.1038/nri3477. The role of dendritic cells in autoimmunity Dipyaman Ganguly1,2,*, Stefan Haak1,3,*, Vanja Sisirak1,*, and Boris Reizis1 1Department of Microbiology and Immunology, Columbia University Medical Center, New York, New York 10032, USA 2Division of Cancer Biology and Inflammatory Disorders, CSIR-Indian Institute of Chemical Biology, Kolkata, West Bengal 700 032, India 3Center for Allergy and Environment (ZAUM), Technical University and Helmholtz Centre München, 80802 Munich, Germany Abstract Dendritic cells (DCs) initiate and shape both the innate and adaptive immune responses. -

Finally 89 Candidates Selected for Admission Placement Opportunities
Department of Biotechnology Ministry of Science & Technology Government of India Annual Report 2018-2019 “Attaining new heights in biotechnology research, shaping biotechnology into a premier precision tool of the future for creation of wealth and ensuring social justice – specially for the welfare of the poor” Composition of the Committee for compilation of Annual Report 2018-19 1. Dr. Alka Sharma, Scientist G – Chairperson 2. Dr. Sandhya Shenoy, Scientist E – Member 3. Dr. Sangita Kasure, Scientist E – Member 4. Dr. Manoj Singh Rohilla, Scientist E – Member 5. Dr. Onkar Nath Tiwari, Scientist E – Member 6. Dr. Padma Singh, Scientist D – Member 7. Shri Mahesh Kumar AD(OL) – Member 8. Shri. J. K. Dora, US(A) – Member 9. Dr. Nitin Kumar Jain, Scientist E – Member Secretary ANNUAL REPORT 2018 - 19 Department of Biotechnology Ministry of Science & Technology Government of India DEPARTMENT OF BIOTECHNOLOGY 1 ANNUAL REPORT 2018-19 2 DEPARTMENT OF BIOTECHNOLOGY Contents 111 OVERVIEW 555 222 BUILDING CAPAAACITIES : HUMAN RESOURCE DEVELOPMENT, TRAININGS AND WORKSHOPS 12 333 RESEARCH & DEVELOPMENT, DEMONSTRATION AND TRANSLATION ACTIVITIES 32 - AGRICULTURE AND ALLIED AREAS 33 - BIOENERGY, BIORESOURCES AND ENVIRONMENT 555111 - HEALTHCARE AND MEDICAL BIOTECHNOLOGY 67 - KNOWLEDGE GENERATION, DISCOVERY RESEARCH, NEW TOOLS AND TECHNOLOGIES 92 444 BUILDING A VIBRANT ECOSYYYSSSTEM : CONNECTING UNIVERSITY RESEARCH AND INDUSTRYYY 103 - RESEARCH RESOURCES, SERVICE FACILITIES AND PLATFORMS 104 - BIO-TECHNOLOGY SCIENCE CLUSTERS 111000777 555 PROMOTING ENTREPRENUERSHIP -
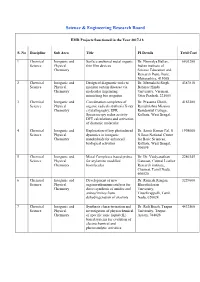
List of Projects Funded by SERB During 2017-18
Science & Engineering Research Board EMR Projects Sanctioned in the Year 2017-18 S. No Discipline Sub Area Title PI Details Total Cost 1 Chemical Inorganic and Surface anchored metal organic Dr. Nirmalya Ballav, 6803280 Science Physical thin film devices Indian institute of Chemistry Science Education and Research Pune, Pune, Maharashtra, 411008 2 Chemical Inorganic and Design of diagnostic tools to Dr. Meenakshi Singh, 4387810 Science Physical monitor certain diseases via Banaras Hindu Chemistry molecular imprinting University, Varanasi, mimicking bio recgnitor Uttar Pradesh, 221005 3 Chemical Inorganic and Coordination complexes of Dr. Prasanta Ghosh, 4163280 Science Physical organic radicals systhesis X-ray Ramakrishna Mission Chemistry crystallography, EPR Residential College, Spectroscopy redox activity Kolkata, West Bengal, DFT calculations and activation of diatomic molecular 4 Chemical Inorganic and Exploration of key photinduced Dr. Samir Kumar Pal, S 1998000 Science Physical dyanmics in inorganic N Bose National Centre Chemistry nanohybrids for enhanced for Basic Sciences, biological activities Kolkata, West Bengal, 700098 5 Chemical Inorganic and Metal Complexes based probes Dr. Dr. Vaidyanathan 2286145 Science Physical for arylamine modified Ganesan, Central Leather Chemistry biomlecules Research institute, Chennai, Tamil Nadu, 600020 6 Chemical Inorganic and Development of new Dr. Ramesh Rengan, 3229600 Science Physical organoruthenium catalysis for Bharathidasan Chemistry direct synthesis of amides and University, anines/imines from Tiruchirappalli, Tamil dehydrogenation of alcohols Nadu, 620024 7 Chemical Inorganic and Synthesis characterization and Dr. Ruli Borah, Tezpur 4432560 Science Physical investigation of physiochemical University, Tezpur, Chemistry of specific ionic liquid (IL) Assam, 784028 based systems for evalution of electrochemical and photocatalytic activities 8 Chemical Inorganic and A Comparative study on Dr. -

NASI Scopus Young Scientist Award in 2014
NASI SCOPUS th YOUNG 12 SCIENTIST AWARDS 2018 Supporting India’s ambition to become a "Knowledge Superpower" NASI-SCOPUS Young Scientist Awards 2018 About Scopus Scopus is the largest abstract and citation database of peer-reviewed literature: scientific journals, books and conference proceedings. Delivering a comprehensive overview of the world’s research output in the fields of science, technology, medicine, social sciences, and arts and humanities, Scopus features smart tools to track, analyze and visualize research. As research becomes increasingly global, interdisciplinary and collaborative, you can make sure that critical research from around the world is not missed when you choose Scopus. “Speed is very important...I can easily identify what I need to know, read it, digest it, and move on to the next one.” James, Research Pathologist, Medical Device R&D, Scopus user “Scopus informs every phase of the editorial process. I would not want to do this job without it, and I intend to continue using it throughout my career.” William, Professor of Economics, University of Tennessee “...[Scopus] will allow us to analyze a deeper range of research activity from a wider range of institutions than present, including those institutions from emerging economies that account for a growing portion of the world’s research output and which have shown a great hunger for THE’s trusted global performance metrics.” THE Managing Director Trevor Barratt For more information, please visit: http://www.elsevier.com/solutions/scopus 1 NASI - SCOPUS YOUNG SCIENTIST AWARDS 2018 India, with its knowledge base, is all set to become the next scientific super power. -

Annual Report 2016-2017
CSIR-IICB CSIR-Indian Institute of Chemical Biology Annual Report 2016-17 #$%&' ! " ! #$%&' ( ! " ! #$%&' ( Courtesy : Dr. Suvendra Nath Bhattacharya Annual Report 2016-17 Jadavpur Campus Salt Lake Campus CSIR-Indian Institute of Chemical Biology 4, Raja S. C. Mullik Road, Jadavpur, Kolkata - 700 032, India Cancer Biology & Project Monitoring Inflammatory Disorder & Evaluation Infectious Diseases Art & & Immunology Photography Cell Biology Business & Physiology Development Group Molecular Genetics ISTAD Organic & Medicinal Chemistry IPM Cell Structural Biology & Bio-informatics P&I R & D P&I - PME & BDG Divisions Division Animal House Electrical Engineering Library & Documentation Maintenance CSIR Electrical Instrumentation IICB Central Architecture Facilities & Civil Engineering Kolkata Infrastructure Computer Maintenance (Civil) Human Resource Group Administration Director’s Finance & Secretariate Accounts General Stores & Administration Purchase Establishment Bill General Section Store R & C Cash Foreign Section Section Purchase Security & Receipts & Indigenous Maintenance Despatch Purchase Works & Hindi Cell Services Contents Director’s Report 005 Cell Biology & Physiology Division 009 Infectious Diseases & Immunology Division 027 Cancer Biology & Inflammatory Disorders Division 037 Organic & Medicinal Chemistry Division 059 Molecular Genetics Division 079 Structural Biology & Bioinformatics Division 085 Network Projects in 12th Five year Plan (2012-17) 105 Publication -

Department of Bio Technology
DEPARTMENT OF BIO TECHNOLOGY List of Ongoing Projects as on 08/07/2016 S.No. Title Project Investigator/Institute Date of Duration Total Cost Sanction (In Rs.) Agriculture Biotechnology – I 1 Programme Support for R&D in agricultural Prof. Anil Kumar Gupta 11/05/2012 5 Years 0 Month 27090000 Biotechnology-phase-II Programme at G.B. Pant G.B. Pant University of Agric. & Tech. University of Agriculture and Technology, Pant Pantnagar Pant Nagar, Uttarakhand- Nagar [BT/PR5031/AGR/2/850/2012] 263128 2 Program Support for Research and Development in Prof. R samiyappan 11/05/2012 5 Years 0 Month 31553004 Agricultural Biotechnology-Phase-II at Tamil Nadu Agricultural University TNAU,Coimbatore [BT/PR5095/AGR/2/847/2012] Coimbatore, Tamilnadu-641003 3 Characterization and molecular mapping of aphid Dr. Beant Singh 20/03/2015 3 Years 0 Month 5147600 (Rhopalosiphum maidis Fitch.) resistance in barley. Punjab Agricultural University [BT/PR9656/AGR/2/870/2013] Ludhiana, Punjab-141004 4 Emergence of Tobacco streak virus infecting Cotton Dr. Renukadevi P 20/04/2015 3 Years 0 Month 4970400 : Investigations on transmission, spread and Tamil Nadu Agricultural University symptom remission. Coimbatore, Tamilnadu-641003 [BT/PR11474/AGR/2/882/2014] 5 CHALLENGE PROGRAMME ON CHICKPEA Dr. Mukesh Jain 23/11/2015 5 Years 0 Month 78161600 FUNCTIONAL GENOMICS [BT/AGR/CG- National Institute of Plant Genome PhaseII/01/2014] Research New Delhi, Delhi-110067 Agriculture Biotechnology – II 6 Development of rice varieties for kerala with Dr. Jayalekshmy V G 02/08/2013 5 Years 0 Month 7240470 pyramided genes for Resistance to BLB by marker Kerala Agricultural University Thrissur, assisted selection [BT/PR13469/AGR/02/703/2010] Kerala-680656 7 Harnessing favorable QTL of wild and exotic Dr. -

Swarnajayanti Fellowship- 2016-17
SwarnaJayanti Fellowship- 2016-17 Applications Received from Eligible Candidates (Applicant may inform if there is any mistake or provide missing information to [email protected] & [email protected] before 31 st May, 2017) LIFE SCIENCES S.No Temporary Applicant Name & Address Email -id Title of the Proposed Reference No. Project Proposal 1. DST/SJF/LS - Dr. Lokesh Gambhir gambhir.lokesh@gmail Isolation and 01/2016-17 Department of Life Sciences .com Characterization of Shri Guru Ram Rai Institute of Xanthine oxidase Technology and Sciences inhibitors from (SGRRITS) endophytic fungi as Patel Nagar prophylaxis for Gout Dehradun-248001 Uttarakhand 2. DST/SJF/LS - Dr. Syed G Dastager [email protected], Improving the 02/2016-17 NCIM Resource Center syed_micro@rediffmail livelihoods of Farmers CSIR National Chemical .com, through superabsorbent Laboratory [email protected] entrapped PGPR for Pune-411008 sustainable agriculture Maharashtra in drought conditions. 3. DST/SJF/LS - Dr. Anurag Jyoti [email protected], Developm ent of 03/2016-17 Amity Institute of [email protected] Nanomaterials based Biotechnology, device for targeted Amity University detection and Gwalior, Opposite-Central potothrmal killing of Potato Research Station Enterotoxigenic Maharajpur, Airport Road Escherichia coli Gwalior-474005 Madhya Pradesh 4. DST/SJF/LS - Dr. Shailendra Pratap Singh [email protected], Study of bolA family 04/2016-17 Department of Botany [email protected] gene in cyanobacteria: Institute of Science, Banaras Bioinformatics analyses Hindu University and functional Varanasi-221005 characterization Uttar Pradesh 5. DST/SJF/LS - Dr. Sanjeev Kumar [email protected] Standardization of 05/2016-17 Department of Vegetable production technology Science for microgreens: A new ASPEE College of Horticulture class of edibles highly & Forestry enriched with bio-active Navsari Agricultural compounds University Navsari-396 450 Gujarat 6.