Insights from a Pan India Sero- Epidemiological Survey
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CDRI Awards 2021 for Excellence in Drug Research
CDRI AWARDS for Excellence in Drug Research 2021 Invitation for Nominations / Applications Instituted by the CSIR-Central Drug Research Institute, Lucknow – 226 031 CDRI AWARDS for Excellence in Drug Research Preamble The CSIR-Central Drug Research Institute (CDRI), Lucknow, was seventh in the chain of CSIR labs that were established in India right after independence India with an aim for technological independence of the Nation. CSIR-CDRI was broadly mandated with the task to revolutionise the pharmaceutical sector, which was almost non-existent at that time for accessibility and affordability of drugs. Today, CSIR-CDRI has evolved into a unique institution possessing end-to-end expertise in the domain of new drug development –its human resource and infrastructure today is second to none. Besides being the breeding ground of huge highly trained human resource for the drug development and manufacturing, out of 20 new drugs discovered, developed and approved in the post-independent India, 11 (8 synthetic + 3 phytopharmaceuticals) are contributions of CSIR-CDRI, Lucknow. These include Centchroman, Arteether, Centbucridine, Gugulipid, Bacosides Enriched Standardized Extract of Bacopa, etc. Centchroman (Ormeloxifene), a non-steroidal contraceptive with almost no toxicity, is now part of the National Family Program, whereas α-β Arteether (antimalarial) is included in the National Malaria Program. However, from the National front, new drug discovery and development is still in its infancy in India. India is a leader in global generic pharmaceuticals manufacturing and is being called as Pharmacy of the developing world, however, many generics manufactured in India are at the end of their respective product life cycles. -

Siiiiimv« ! W^9
I. » ■ v l - siiiiiMv« ! W^9 mffl: ~ i- ■ •• m . !. I ! i ■ I '• Ad Majorem Dei Gloriam (For the Greater Glonj of God) ST IGNATIUS OF LOYOLA 0491-1556) THE LOYOLITE 2016 1 Editorial Board Publisher: FrDevassy Paul, SJ Principal, Loyola School Thiruvananthapuram Staff Editors: Mr Ajaya Kumar Mrs Anitha Kumari S.I. Mrs Ashwathy Pradeep Mrs Aysha K.P. Mrs Baijayantee Bhattacharya Mrs Brinda Nair Mrs Mar}' Mathew Principal's Annual Report - 2016....... 4 Mr Remesan P.L. 9 ; English Section - Juniors...................... Student Editors: Leaders' Laurels.................................... 18 Master Francis Naveen A. (XI A) 20 Master Viswajit Vinod Nair (XI A) Report- LA Fest..................................... Master Nived Chandrasekharan (XIB) Winners and their Win......................... 22 l Master Richy Yesudas (XI B) Report - Basketball Tournament........ 26 Master Sai Ganesh R. (XI C) Master Vivek Wilkins (XI C) A Farewell Note.................................... 28 : : Master Shyam Raja Puthiyakovilakam (XI D) Report - Youth Festival....................... 29 Master Soorya S. Padmanabhan (XI D) ■ We shall meet on that beautiful shore. 31 : Photo Compilation: English Section - Seniors...................... 33 Mrs Sindhu Sarma Master Abhijith S. Raj (IX B) Artists' Spread....................................... 65 Master Achyuth P. (IX B) Report: NCC.......................................... 68 I 69 Photography: Malayalam Section................................ Mr Vinod Kumar Sevanathinte Ara Noottandu............. 77 Focus, Pettah -
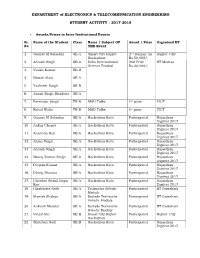
ETC Student Activity 2017-18.Pdf
DEPARTMENT of ELECTRONICS & TELECOMMUNICATION ENGINEERING STUDENT ACTIVITY : 2017-2018 Awards/Prizes in Inter Institutional Events Sr. Name of the Student Class Name / Subject OF Award / Prize Organized BY No THE Event 1. Gaurav M Salaskar SE-A Smart City Rajkot 2nd Runner up Rajkot City Hackathon Rs.50,000/- 2. Adrash Singh SE-A India International 2nd Prize IIT Madras Science Festival Rs:20,000/- 3. Vinish Kumar SE-B 4. Nawab Alam SE-A 5. Yashveer Singh SE-B 6. Akash Singh Bhadoria SE-A 7. Parvinder Singh TE-B MAD Talks 1st prize PICT 8. Rahul Walia TE-B MAD Talks 1st prize PICT 9. Gaurav M Salaskar SE-A Hackathon Kota Participated Rajasthan Digifest 2017 10. Ankur Chopra SE-A Hackathon Kota Participated Rajasthan Digifest 2017 11. Aravinda Hati SE-A Hackathon Kota Participated Rajasthan Digifest 2017 12. Aman Singh SE-A Hackathon Kota Participated Rajasthan Digifest 2017 13. Adrash Singh SE-A Hackathon Kota Participated Rajasthan Digifest 2017 14. Neeraj Kumar Singh SE-A Hackathon Kota Participated Rajasthan Digifest 2017 15. Deepak Kumar SE-A Hackathon Kota Participated Rajasthan Digifest 2017 16. Dhiraj Sharma SE-A Hackathon Kota Participated Rajasthan Digifest 2017 17. Dhenbre Sushil Bapu SE-A Hackathon Kota Participated Rajasthan Rao Digifest 2017 18. Hitabarata Nath SE-A Techniche Robotic Participated IIT Guwahati Module 19. Manish Shakya SE-A Esclade Techniche Participated IIT Guwahati Robotic Module 20. Ankush Mandal SE-A Esclade Techniche Participated IIT Guwahati Robotic Module 21. Vatsal Rai SE-B Smart City Rajkot Participated Rajkot City Hackathon 22. Shobhan Nath SE-B Hackathon Kota Participated Rajasthan Digifest 2017 DEPARTMENT of ELECTRONICS & TELECOMMUNICATION ENGINEERING STUDENT ACTIVITY : 2017-2018 Sr. -

List of Life Members As on 20Th January 2021
LIST OF LIFE MEMBERS AS ON 20TH JANUARY 2021 10. Dr. SAURABH CHANDRA SAXENA(2154) ALIGARH S/O NAGESH CHANDRA SAXENA POST HARDNAGANJ 1. Dr. SAAD TAYYAB DIST ALIGARH 202 125 UP INTERDISCIPLINARY BIOTECHNOLOGY [email protected] UNIT, ALIGARH MUSLIM UNIVERSITY ALIGARH 202 002 11. Dr. SHAGUFTA MOIN (1261) [email protected] DEPT. OF BIOCHEMISTRY J. N. MEDICAL COLLEGE 2. Dr. HAMMAD AHMAD SHADAB G. G.(1454) ALIGARH MUSLIM UNIVERSITY 31 SECTOR OF GENETICS ALIGARH 202 002 DEPT. OF ZOOLOGY ALIGARH MUSLIM UNIVERSITY 12. SHAIK NISAR ALI (3769) ALIGARH 202 002 DEPT. OF BIOCHEMISTRY FACULTY OF LIFE SCIENCE 3. Dr. INDU SAXENA (1838) ALIGARH MUSLIM UNIVERSITY, ALIGARH 202 002 HIG 30, ADA COLONY [email protected] AVANTEKA PHASE I RAMGHAT ROAD, ALIGARH 202 001 13. DR. MAHAMMAD REHAN AJMAL KHAN (4157) 4/570, Z-5, NOOR MANZIL COMPOUND 4. Dr. (MRS) KHUSHTAR ANWAR SALMAN(3332) DIDHPUR, CIVIL LINES DEPT. OF BIOCHEMISTRY ALIGARH UP 202 002 JAWAHARLAL NEHRU MEDICAL COLLEGE [email protected] ALIGARH MUSLIM UNIVERSITY ALIGARH 202 002 14. DR. HINA YOUNUS (4281) [email protected] INTERDISCIPLINARY BIOTECHNOLOGY UNIT ALIGARH MUSLIM UNIVERSITY 5. Dr. MOHAMMAD TABISH (2226) ALIGARH U.P. 202 002 DEPT. OF BIOCHEMISTRY [email protected] FACULTY OF LIFE SCIENCES ALIGARH MUSLIM UNIVERSITY 15. DR. IMTIYAZ YOUSUF (4355) ALIGARH 202 002 DEPT OF CHEMISTRY, [email protected] ALIGARH MUSLIM UNIVERSITY, ALIGARH, UP 202002 6. Dr. MOHAMMAD AFZAL (1101) [email protected] DEPT. OF ZOOLOGY [email protected] ALIGARH MUSLIM UNIVERSITY ALIGARH 202 002 ALLAHABAD 7. Dr. RIAZ AHMAD(1754) SECTION OF GENETICS 16. -

Adipose Recruitment and Activation of Plasmacytoid Dendritic Cells Fuel Metaflammation’
Diabetes Page 2 of 61 Adipose recruitment and activation of plasmacytoid dendritic cells fuel metaflammation Amrit Raj Ghosh1, Roopkatha Bhattacharya1, Shamik Bhattacharya1, Titli Nargis2, Oindrila Rahaman1, Pritam Duttagupta1, Deblina Raychaudhuri1, Chinky Shiu Chen Liu1, Shounak Roy1, Parasar Ghosh3, Shashi Khanna4, Tamonas Chaudhuri4, Om Tantia4, Stefan Haak5, Santu Bandyopadhyay1, Satinath Mukhopadhyay6, Partha Chakrabarti2 and Dipyaman Ganguly1*. Divisions of 1Cancer Biology & Inflammatory Disorders and 2Cell Biology & Physiology, CSIR- Indian Institute of Chemical Biology, Kolkata, India; 4ILS Hospitals, Kolkata, India; 5Zentrum Allergie & Umwelt (ZAUM), Technical University of Munich and Helmholtz Centre Munich, Munich, Germany; Departments of 3Rheumatology and 6Endocrinology, Institute of Postgraduate Medical Education & Research, Kolkata, India. *Corresponding author: Dipyaman Ganguly, Division of Cancer Biology & Inflammatory Disorders, CSIR-Indian Institute of Chemical Biology, 4 Raja S C Mullick Road, Jadavpur, Kolkata, West Bengal, India, 700032. Phone: 91 33 24730492 Fax: 91 33 2473 5197 Email: [email protected] Running title: PDCs and type I interferons in metaflammation Word count (Main text): 5521 Figures: 7, Table: 1 1 Diabetes Publish Ahead of Print, published online August 25, 2016 Page 3 of 61 Diabetes ABSTRACT In obese individuals the visceral adipose tissue (VAT) becomes seat of chronic low grade inflammation (metaflammation). But the mechanistic link between increased adiposity and metaflammation remains largely -

National Institutional Ranking Framework
National Institutional Ranking Framework Ministry of Human Resource Development Government of India Welcome to Data Capturing System: OVERALL Submitted Institute Data for NIRF'2020' Institute Name: ACADEMY OF SCIENTIFIC & INNOVATIVE RESEARCH [IR-O-U-0713] Sanctioned (Approved) Intake Academic Year 2018-19 2017-18 2016-17 2015-16 2014-15 2013-14 PG [1 Year Program(s)] 17 - - - - - PG [2 Year Program(s)] 136 65 - - - - Total Actual Student Strength (Program(s) Offered by Your Institution) (All programs No. of Male No. of Female Total Students Within State Outside State Outside Economically Socially No. of students No. of students No. of students No. of students of all years) Students Students (Including male (Including male Country Backward Challenged receiving full receiving full receiving full who are not & female) & female) (Including male (Including male (SC+ST+OBC tuition fee tuition fee tuition fee receiving full & female) & female) Including male reimbursement reimbursement reimbursement tuition fee & female) from the State from Institution from the Private reimbursement and Central Funds Bodies Government PG [1 Year 6 3 9 2 7 0 0 2 0 0 0 2 Program(s)] PG [2 Year 53 56 109 48 61 0 1 42 1 1 0 41 Program(s)] Placement & Higher Studies PG [1 Years Program(s)]: Placement & higher studies for previous 3 years Academic Year No. of first year No. of first year Academic Year No. of students graduating in minimum No. of students Median salary of No. of students students intake in the students admitted in stipulated time placed placed selected for Higher year the year graduates(Amount in Studies Rs.) 2016-17 20 20 2016-17 17 5 319000(Rupees Three 9 Lakhs Nineteen Thousand Only) 2017-18 16 16 2017-18 16 10 330000(Rupees Three 2 Lakhs Thirty Thousand Only) 2018-19 17 9 2018-19 9 2 325000(Rupees Three 4 Lakhs Twenty Five Thousand Only) PG [2 Years Program(s)]: Placement & higher studies for previous 3 years Academic Year No. -

Year Book 2018 Year Book 2018
YEAR BOOK 2018 YEAR BOOK 2018 WEST BENGAL ACADEMY OF SCIENCE AND TECHNOLOGY CSIR-Indian Institute of Chemical Biology Jadavpur YEAR BOOK Kolkata 700 032 Registered under the West Bengal Act XXVI of 1961 (S/65001 of 1990-91) 2018 PAN – AAATW0707E Published by : Prof. Satyabrata Pal, Elected Member, ISI, FRSS Formerly, Dean, Post Graduate Studies, BCKV and Honorary Visiting Professor, ISI, Kolkata Editor, West Bengal Acadepmy of Science and Technology Assisted by : Dr. Arun Bandyopadhyay, Ph.D. Chief Scientist, CSIR-IICB, Kolkata-700 032 Secretary, West Bengal Academy of Science and Technology WAST Secretariat CSIR-Indian Institute of Chemical Biology 4, Raja S. C. Mullick Road WEST BENGAL Jadavpur, Kolkata 700 032 A C Telephone: (033) 2499-5796 A W A D e-mail: [email protected] E M Website: http://www.iicb.res.in/wast/index.html S T Y SCIENCE Printed by : WEST BENGAL ACADEMY OF SCIENCE AND TECHNOLOGY Creative Data Centre Registered Office : CSIR-Indian Institute of Chemical Biology 58/32, Prince Anwar Shah Road 4, Raja S. C. Mullick Road, Jadavpur Kolkata- 700 045 Kolkata 700 032 E-mail: [email protected] 1 2 YEAR BOOK 2018 YEAR BOOK 2018 AD-HOC Committee (1986-1989) Contents 1. Professor Sushil Kumar Mukherjee : Chairman 2. Professor Syama Pada Sen Introduction 5 3. Professor Asok Ghosh Memorandum of Association 6 4. Dr. Satyesh Chandra Pakrashi Rules and Regulations 9 Approved Amendments–I 25 5. Professor Subodh Kumar Roy Approved Amendments–II 29 6. Professor Asok Kumar Barua Past Office Bearers 34 7. Professor Nityananda Saha Council : 2016-2018 37 Sectional Committees : 2016-2018 39 8. -
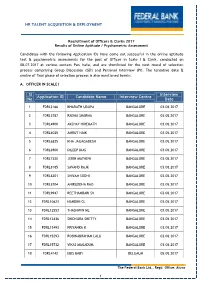
Sl No Application ID Candidate Name Interview Centre Interview Date HR TALENT ACQUISITION & DEPLOYMENT
HR TALENT ACQUISITION & DEPLOYMENT Recruitment of Officers & Clerks 2017 Results of Online Aptitude / Psychometric Assessment Candidates with the following Application IDs have come out successful in the online aptitude test & psychometric assessments for the post of Officer in Scale I & Clerk, conducted on 08.07.2017 at various centers Pan India, and are shortlisted for the next round of selection process comprising Group Discussion (GD) and Personal Interview (PI). The tentative date & centre of final phase of selection process is also mentioned herein: A. OFFICER IN SCALE I Sl Interview Application ID Candidate Name Interview Centre No Date 1 FDRL3166 BHARATH UDUPA BANGALORE 03.08.2017 2 FDRL3787 RADHA SHARMA BANGALORE 03.08.2017 3 FDRL4900 AKSHAY HIREMATH BANGALORE 03.08.2017 4 FDRL6025 AMRUT NAIK BANGALORE 03.08.2017 5 FDRL6825 KHA JAIJAGADESH BANGALORE 03.08.2017 6 FDRL6900 DILEEP DIAS BANGALORE 03.08.2017 7 FDRL7320 JERIN MATHEW BANGALORE 03.08.2017 8 FDRL8185 SAVAND RAJK BANGALORE 03.08.2017 9 FDRL8201 SHIVAM SODHI BANGALORE 03.08.2017 10 FDRL8704 ANIRUDDHA RAO BANGALORE 03.08.2017 11 FDRL9947 REETHAMBARI SV BANGALORE 03.08.2017 12 FDRL10623 NANDINI CL BANGALORE 03.08.2017 13 FDRL12553 THASHWIN ML BANGALORE 03.08.2017 14 FDRL12836 SINDHURA SHETTY BANGALORE 03.08.2017 15 FDRL13493 PRIYANKA K BANGALORE 03.08.2017 16 FDRL15293 ROBINABRAHAM LALU BANGALORE 03.08.2017 17 FDRL15732 VIKAS MAGADUM BANGALORE 03.08.2017 18 FDRL4142 EBIS BABY BELGAUM 05.08.2017 The Federal Bank Ltd., Regd. Office: Aluva 1 19 FDRL5411 SHREYA S BELGAUM 05.08.2017 -

Football Heroes
NorthEast United FC Atlético de Kolkata Mumbai City FC Delhi Dynamos Owners: John Abraham and Shillong Lajong Owners: Kolkata Games and Sports Pvt. Ltd Owners: Ranbir Kapoor and Bimal Parekh Owners: DEN Networks Coach: Ricki Herbert (partly owned by Atlético Madrid) Coach: Peter Reid Coach: Harm van Veldhoven Key players: Miguel Garcia, Cornell Glen Coach: Antonio López Habas Key players: Syed Rahim Nabi, Fredrik Key players: Morten Skoubo, Steven Dias Player to watch out for: Joan Capdevilla Key players: Climax Lawrence, Borja Ljungberg Fernández Player to watch out for: Alessandro Del Player to watch out for: Nicolas Anelka Piero A serious protege in his younger days, Nicolas Anelka A Juventus legend and one of the greatest strikers of his was first spotted by Arsenal manager Arsene Wenger in generation, Alessandro Del Piero will play for Delhi 1997 at the tender age of 17. He went on to make Dynamos in ISL. Described by Diego Maradona as “ a appearances for numerous big clubs across Europe, man who feels football in his soul”,the Italian was the including Real Madrid, Liverpool and Chelsea. Often complete striker during his illustrious career, possessing loathed for his sulking ways, Anelka moved to Shanghai a solid range of passing, vision and dribbling ability. A Shenhua in 2012, before making a forgettable return to World Cup winner with Italy in 2006, he is Juventus’ England with West Bromwich Albion last season. He will record goal scorer with 290 goals. be FC Mumbai City’s marquee player. REUTERS A MEMBER OF SPAIN’S GOLDEN GENERATION THAT WON EURO 2008 and the 2010 World Cup, Joan Capdevilla played a pivotal role at left back in both those triumphs. -

1 Psi News Letter January-2019
PSI NEWS LETTER 1 JANUARY-2019 CONTENTS SECTION NAME PAGE NO’S 1. EDITOR’S MESSAGE............................................... ......................................4 2. REPORT OF PSI, 10TH ANNUAL MEETING.................................................5 - 11 3. CPG WORKSHOP, IIT BOMBAY...................................................................12 - 14 4. JPP: “SPRINGS” TO THE INTERNATIONAL STAGE ...............................15 5. MRM CONSORTIUM: A PSI INITIATIVE................................................16 6. RESEARCH UPDATES.................... .............................................................. 17-19 7. PSI - 2018 AWARD WINNERS.......... .............................................................20-26 8. UPCOMING WORKSHOPS & EVENTS........................................................27-31 PSI NEWS LETTER 2 JANUARY-2019 PSI EXECUTIVE COMMITTEE President Member Dr. Utpal S Tatu Dr. Rakesh K Mishra Vice President Dr. Abhijit Chakrabarti Dr. Subhra Chakraborty Dr. Shantanu Sengupta Dr. Arun Bandyopadhyay Dr. K Dharmalingam Gen. Secretary Dr. Kalpana Bhargava Dr. Mahesh J Kulkarni Dr. Niranjan Chakraborty Dr. Amol R Suryawanshi Treasurer Dr. M.V Jagannadham Dr. Suman S. Thakur Dr. Harsha Gowda Jt. Secretary Dr. Srikanth Rapole Dr. Sanjeeva Srivastava Dr. Suman Kundu Past President of PSI Dr. Ashok Kumar Mohanty Dr. Ravi Sirdeshmukh Dr. Sharmila Chattopadhyay (Founder President, EC member) Dr. Debasis Dash Dr. Surekha Zingde (Immediate Past President, ex officio member of EC) PSI NEWS LETTER 3 JANUARY-2019 -

Awardees of National Bioscience Award for Career Development
AWARDEES OF NATIONAL BIOSCIENCE AWARD FOR CAREER DEVELOPMENT Awardees for the year 2016 1. Dr. Mukesh Jain, Associate Professor, School of Computational and Integrative Sciences, Jawaharlal Nehru University, New Delhi-110067 2. Dr. Samir K. Maji, Associate Professor, Indian Institute of Technology, Powai, Mumbai- 400076 3. Dr. Anindita Ukil, Assistant Professor, Calcutta University, Kolkata 4. Dr. Arnab Mukhopadhyay, Staff Scientist V, National Institute of Immunology, Aruna Asaf Ali Marg, New Delhi- 110067 5. Dr. Rohit Srivastava, Professor, Indian Institute of Technology, Bombay, Mumbai- 400076 6. Dr. Pinaki Talukdar, Associate Professor, Indian Institute of Science Education and Research, Dr. Homi Bhabha Road, Pashan, Pune- 7. Dr. Rajnish Kumar Chaturvedi, Senior Scientist, CSIR- Indian Institute of Toxicology Research, Lucknow-226001 8. Dr. Jackson James, Scientist E-II, Neuro Stem Cell Biology Lab, Neurobiology Division, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, Kerala- 695014 Awardees for the year 2015 1. Dr. Sanjeev Das, Staff Scientist-V, National Institute of Immunology, New Delhi 2. Dr. Ganesh Nagaraju, Assistant Professor, Department of Biotechnology, Indian Institute of Science, Bangalore- 5600012. 3. Dr. Suvendra Nath Bhattacharya, Principal Scientist, CSIR- Indian Institute of Chemical Biology, Kolkata- 700032 4. Dr. Thulasiram H V, Principal Scientist, CSIR-National Chemical Laboratory, Pune- 411008. 5. Dr. Pawan Gupta, Principal Scientist, Institute of microbial Technology, Chandigarh- 160036. 6. Dr. Souvik Maiti, Principal Scientist, CSIR-Institute of Genomics and Integrative Biology, Delhi- 110025. 7. Dr. Pravindra Kumar, Associate Professor, Department of Biotechnology, IIT, Roorkee- 247667. 8. Dr. Anurag Agrawal, Principal Scientist, CSIR-Institute of Genomics and Integrative Biology, Delhi- 110025 9. Dr. Gridhar Kumar Pandey, Professor, Department of Plant Molecular Biology, University of Delhi South Campus, New Delhi- 110067 10. -

National Bioscience Awards for Career Development
AWARDEES OF NATIONAL BIOSCIENCE AWARDS FOR CAREER DEVELOPMENT Awardees for the year 2012 1. Dr. Kaustuv Sanyal, Associate Professor, Molecular Mycology Laboratory, Molecular Biology & Genetics Unit, Jawaharlal Nehru Centre for Advance Scientific Research, Jakkur P.O. Bangalore 560064 2. Dr Naval Kishore Vikram, Associate Professor, Department of Medicine, All India Institute of Medical Sciences (AIIMS), Ansari Nagar, New Delhi- 110029 3. Dr. Aditya Bhushan Pant, Senior Scientist & In-charge, In Vitro Toxicology Laboratory, Indian Institute of Toxicology Research, PO Box: 80, MG Marg, Lucknow 226001 (UP) India 4. Dr. Subrata Adak, Senior Scientist, Indian Institute of Chemical Biology; 4, Raja S.C. Mullick Road, Kolkata-700032 5. Dr. Durai Sundar, Assistant Professor, Dept of Biochemical Engineering & Biotechnology, Indian Institute of Technology (IIT) Delhi, Hauz Khas, New Delhi – 110016 6. Dr S Venkata Mohan, Senior Scientist, Bioengineering and Environmental Centre (BEEC) CSIR-Indian Institute of Chemical Technology, Hyderabad-500 607 7. Dr. Munia Ganguli, Scientist E-I, CSIR-Institute of Genomics & Integrative Biology, Mall Road,New Delhi 110 007 8. Dr. Asad U Khan, Associate Professor & Coordinator/Head of Biotechnology Department, A.M.U, Interdisciplinary Biotechnology Unit, A.M.U., Aligarh 202002 9. Dr. Sathees C. Raghavan, Assistant Professor, Department of Biochemistry, Indian Institute of Science, Bangalore 560 012 10. Dr. Vidita A. Vaidya, Associate Professor, Department of Biological Sciences, Tata Institute of Fundamental Research, 1, Homi Bhabha Road, Colaba, Mumbai - 400005 Awardees for the year 2011 1. Dr. M. M. Parida, Scientist-F, Joint Director, Division of Virology Defence R & D Establishment, DRDE, DRDO, Ministry of Defence, Jhansi Road, Gwalior- 474002 2.