Draft Screening Assessment Is Based Are Summarized Below
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Technical/Application Article 02 Version 1.10 22Nd January 2018 WRH/FD
Technical/Application Article 02 Version 1.10 22nd January 2018 WRH/FD Ion Science PID Response Factors PID Response Photoionisation Detectors (PIDs) respond to a broad range of organic and a few inorganic gaseous and volatile chemicals (‘volatiles’). In order for PID to respond to a volatile, the photon energy of the lamp must be greater than its ionisation energy (IE). Ion Science PIDs are available with lamps emitting light of maximum energy of 10.0 eV, 10.6 eV, and 11.7 eV. This Technical Article lists the response factors (‘RF’s’) for over 900 volatiles with PID incorporating these lamps. The RF relates the sensitivity of PID to a volatile to the sensitivity to the standard calibration gas isobutylene. The higher the RF, the lower the sensitivity. Isobutylene as Reference Gas Ideally, the PID response to a chemical volatile would be calibrated by using a low concentration of the chemical in air. However, this is often not practical. Isobutylene is then used to calibrate PID, and a Response Factor (RF) used to convert the isobutylene calibrated measurement to a measurement of the target volatile: Concentration of target chemical = isobutylene calibrated measurement x RF For example, the RF of anisole is 0.59 with a 10.6 eV lamp. Therefore, a reading of 10 ppm using an isobutylene-calibrated unit would indicate: Concentration of anisole = 10 ppm x 0.59 = 5.9 ppm In Ion Science detectors, RFs are pre-programmed into a compound library and can be called up to make the PID read out in units of the chemical of interest. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

Fullerene-Acene Chemistry
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 2007 Fullerene-acene chemistry: Part I Studies on the regioselective reduction of acenes and acene quinones; Part II Progress toward the synthesis of large acenes and their Diels-Alder chemistry with [60]fullerene Andreas John Athans University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation Athans, Andreas John, "Fullerene-acene chemistry: Part I Studies on the regioselective reduction of acenes and acene quinones; Part II Progress toward the synthesis of large acenes and their Diels-Alder chemistry with [60]fullerene" (2007). Doctoral Dissertations. 363. https://scholars.unh.edu/dissertation/363 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. FULLERENE-ACENE CHEMISTRY: PART I: STUDIES ON THE REGIOSELECTIVE REDUCTION OF ACENES AND ACENE QUINONES; PART II: PROGRESS TOWARD THE SYNTHESIS OF LARGE ACENES AND THEIR DIELS- ALDER CHEMISTRY WITH [60]FULLERENE VOLUME 1 CHAPTERS 1-5 BY ANDREAS JOHN ATHANS B.S. University of New Hampshire, 2001 DISSERTATION Submitted to the University of New Hampshire in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy m Chemistry May, 2007 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: 3 2 6 0 5 8 6 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. -

Hydrogenation of 2-Methylnaphthalene in a Trickle Bed Reactor Over Bifunctional Nickel Catalysts
The University of Maine DigitalCommons@UMaine Electronic Theses and Dissertations Fogler Library Fall 12-2020 Hydrogenation of 2-methylnaphthalene in a Trickle Bed Reactor Over Bifunctional Nickel Catalysts Matthew J. Kline University of Maine, [email protected] Follow this and additional works at: https://digitalcommons.library.umaine.edu/etd Part of the Catalysis and Reaction Engineering Commons, and the Petroleum Engineering Commons Recommended Citation Kline, Matthew J., "Hydrogenation of 2-methylnaphthalene in a Trickle Bed Reactor Over Bifunctional Nickel Catalysts" (2020). Electronic Theses and Dissertations. 3284. https://digitalcommons.library.umaine.edu/etd/3284 This Open-Access Thesis is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. HYDROGENATION OF 2-METHYLNAPHTHALENE IN A TRICKLE BED REACTOR OVER BIFUNCTIONAL NICKEL CATALYSTS By Matthew J. Kline B.S. Seton Hill University, 2018 A THESIS Submitted in Partial Fulfillment of the Requirements For the Degree of Master oF Science (in Chemical Engineering) The Graduate School The University of Maine December 2020 Advisory Committee: M. Clayton Wheeler, Professor of Chemical Engineering, Advisor Thomas J. Schwartz, Assistant ProFessor oF Chemical Engineering William J. DeSisto, ProFessor oF Chemical Engineering Brian G. Frederick, ProFessor oF Chemistry -

Polypropylene Chemical Resistance Guide Chemical Listing & Ratings
Polypropylene Chemical Resistance Guide Chemical Listing & Ratings hmcpolymers.com POLYPROPYLENE CHEMICAL RESISTANCE About HMC Polymers HMC Polymers is focused on being PP chemical resistance Asia’s number ONE in PP. HMC Polymers’ polypropylene resins, like most HMC PP resins are appreciably affected Some reduction in tensile strength and an polyolens, are highly resistant to solvents and by chlorosulfonic acid and oleum at increase in exibility and elongation-to-break We work in close cooperation with chemicals. room temperature, 98% sulfuric acid, 30% in tension can be expected, depending on our customers to improve existing hydrochloric acid, and 30% hydrogen peroxide the nature and amount of the organic The results of extensive laboratory and actual at 100°C (212°F). They are also affected by 98% medium absorbed. products and develop new resins eld installation tests of polypropylene’s sulfuric acid at 60°C (140°F) and fuming nitric for the rapidly changing markets we chemical resistance are reported in this HMC PP resins have excellent resistance acid and liquid bromine at room temperatures. serve. The company offers a wide downloadable PDF which is periodically to environmental stress-cracking. When Under strain, failure could occur with strong updated. they are tested according to ASTM D1693 range of products suitable for all oxidizing acids at temperatures lower than the brittle fractures that occur with certain major applications and processes, The chemical resistance data presented in this those mentioned. With few exceptions polyethylenes in contact with polar PDF for a range of over 260 chemicals and however, inorganic chemicals produce little or as well as innovative new products organic liquids, detergents, and silicone substances is based on ASTM D543. -
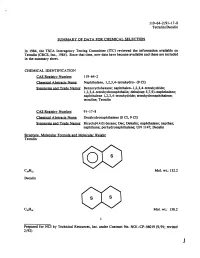
Tetralin/Decalin
119-64-2/91-17-8 Tetralin/Decalin SUMMARY OF DATA FOR CHEMICAL SELECTION In 1984, the TSCA Interagency Testing Committee (lTC) reviewed the information available on Tetralin (CRCS, Inc., 1984). Since that time, new data have become available and these are included in the summary sheet. CHEMICAL IDENTIFICATION CAS Registry Number: 119-64-2 Chemical Abstracts Name: Naphthalene, 1,2,3,4-tetrahydro- (9 CI) Synonyms and Trade Names: Benzocyclohexane; naphthalen-1,2,3,4-tetrahydride; 1,2,3,4-tetrahydronaphthalin; delta(sup 5,7 ,9)-naphthalene; naphthalene 1,2,3,4-tetrahydride; tetrahydronaphthalene; tetraline; Tetralin CAS Registry Number: 91-17-8 Chemical Abstracts Name: Decahydronaphthalene (8 CI, 9 CI) Synonyms and Trade Names: Bicyc1o[4.4.0] decane; Dec; Dekalin; naphthalane; napthan; naphthane; perhydronaphthalene; UN 1147; Decalin Structure. Molecular Formula and Molecular Weight Tetralin Mol. wt.: 132.2 Decal in Mol. wt.: 138.2 1 Prepared for NCI by Technical Resources, Inc. under Contract No. N01-CP-56019 (9/91; revised 2/92) 1 119-64-2/91-17-8 Tetralin/Decalin Chemical and Physical Prooerties: From Budavari (1989) unless otherwise noted. Tetralin Description: Liquid with odor resembling that of a mixture of benzene and menthol Boiling Point: 207.2° C Melting Point: -31 o C Solubility: Insoluble in water; soluble in methanol at 50.6% wtjwt and aniline; very soluble in ether; miscible with petroleum ether, chloroform, and Decalin, ethanol, butanol, acetone and benzene StabilitY: Prolonged, intimate contact with air may cause the formation -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide FIRST EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 1st Edition © 2019 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

TR-561: Tetralin (CASRN 119-64-2) in F344/N Rats and B6C3F1 Mice
NTP TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF TETRALIN (CAS NO. 119-64-2) IN F344/N RATS AND B6C3F1 MICE AND A TOXICOLOGY STUDY OF TETRALIN IN MALE NBR RATS (INHALATION STUDIES) NATIONAL TOXICOLOGY PROGRAM P.O. Box 12233 Research Triangle Park, NC 27709 April 2011 NTP TR 561 NIH Publication No. 11-5902 National Institutes of Health Public Health Service U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES FOREWORD The National Toxicology Program (NTP) is an interagency program within the Public Health Service (PHS) of the Department of Health and Human Services (HHS) and is headquartered at the National Institute of Environmental Health Sciences of the National Institutes of Health (NIEHS/NIH). Three agencies contribute resources to the program: NIEHS/NIH, the National Institute for Occupational Safety and Health of the Centers for Disease Control and Prevention (NIOSH/CDC), and the National Center for Toxicological Research of the Food and Drug Administration (NCTR/FDA). Established in 1978, the NTP is charged with coordinating toxicological testing activities, strengthening the science base in toxicology, developing and validating improved testing methods, and providing information about potentially toxic substances to health regulatory and research agencies, scientific and medical communities, and the public. The Technical Report series began in 1976 with carcinogenesis studies conducted by the National Cancer Institute. In 1981, this bioassay program was transferred to the NTP. The studies described in the Technical Report series are designed and conducted to characterize and evaluate the toxicologic potential, including carcinogenic activity, of selected substances in laboratory animals (usually two species, rats and mice). -
I Application of Hansen Solubility Parameters in Fabrication
Application of Hansen Solubility Parameters in Fabrication of Molecularly Im- printed Polymers for Detection of PAHs from Water Samples By Maryam Jafari A thesis submitted to the School of Graduate Studies in partial fulfillment of the require- ments for the degree of Master of Science Department of Chemistry Memorial University of Newfoundland and Labrador July 2018 St. John’s Newfoundland and Labrador i Abstract Molecularly imprinted polymers (MIPs) are smart polymers for selective recognition of target analytes. This work focuses on developing MIPs for aquatic contaminants, such as polycyclic aromatic hydrocarbons (PAHs) in seawater. MIPs are generally prepared with a monomer, cross-linker, template or pseudo-template, and solvent (porogen). As with most porous adsorbents, MIPs exhibit high surface area and porosity, controlled pore size and mechanical stability. It has been recognized that the solvent system plays a key role in the pore generation, influencing the shape, size and volume of pores in MIPs. Rather than taking a trial-and-error approach, Hansen solubility parameters (HSPs) are used in this work to develop a model to predict the suitability of porogen for the formation of MIP films with specified porosity. Hansen solubility parameters help to predict the ther- modynamic compatibility of a porogen with the prepolymerization components, which is a good estimate of the propensity to form a polymeric network with required characteris- tics. MIPs fabricated using the systematic method based on Hansen solubility parameters were combined with GC-MS to determine the concentration of naphthalene, fluorene, phenan- threne and pyrene in water samples. The porous MIPs were also used to extract and de- termine the concentration of PAHs in produced water which is a byproduct generated along with the production of oil and gas from on shore and offshore platforms. -

Use of High Throughput Assays and Computational Tools; Endocrine Disruptor Screening Program; Notice of Availability and Opportunity for Comment’’
NRDC EPA-HQ-OPPT-2015-0305 August, 2015 Comments from the Natural Resources Defense Council On The Document Titled, ‘‘Use of High Throughput Assays and Computational Tools; Endocrine Disruptor Screening Program; Notice of Availability and Opportunity for Comment’’ To the U.S. Environmental Protection Agency Docket No. EPA-HQ-OPPT-2015-0305 August 18, 2015 Background The Natural Resources Defense Council ("NRDC") is a national, non-profit environmental organization of lawyers, scientists, and other professionals. NRDC presents these comments on behalf of our 1.4 million members and online activists. NRDC does not have any financial interest in the topic of these comments. The endocrine system utilizes highly complex, tightly controlled molecular processes for its optimal functioning in the body. The proper balance of hormones can be synchronized in a variety of ways (including direct protein binding, epigenetic alterations, gene activation and silencing), and is essential across the entirety of the life course. Small changes in the perfectly orchestrated symphony of hormone levels can severely disrupt the harmony necessary for critical windows of development (e.g., fetal development, infanthood, childhood, and adolescence), leaving the body vulnerable to a host of negative health outcomes (such as diabetes, cancer, obesity, and reproductive dysfunction). The last decade has seen an exponential increase in the development of computational, biological, and chemical tools capable of increasing both the number of chemicals analyzed and the pace of chemical toxicity evaluation. These tools, including the EPA Toxicity Forecaster (ToxCast™) and the National Institute of Environmental Health Sciences (NIEHS) Tox21 platforms, have the potential to rapidly generate molecular and cellular data for thousands of chemicals at once, and provide an additional stream of useful information that can aide in regulatory decision-making. -

DMACC Chemical Hygiene Plan
CHEMICAL HYGIENE PLAN DES MOINES AREA COMMUNITY COLLEGE ALL CAMPUSES Last Revision: November 1, 2005 TABLE OF CONTENTS PAGE A. INTRODUCTION...............................................................................................A-1 Regulatory Basis..................................................................................................A-1 Administrative Responsibilities...........................................................................A-1 Required Content of Plan.....................................................................................A-2 B. STANDARD OPERATING PROCEDURES.................................................B-1 Ordering Chemicals .............................................................................................B-1 Receipt and Distribution of Chemicals................................................................B-2 Safe Storage of Chemicals...................................................................................B-3 Safe Use of Chemicals.........................................................................................B-4 Safe Use of Flammable/Combustibles.................................................................B-6 Safe Use of Corrosives.........................................................................................B-7 Safe Use of Reactives ..........................................................................................B-8 Special Procedures for Carcinogens, Reproductive Toxins and Acute Toxins ..............................................................B-9 -

Cracking Fundamentals
Thermal Cracking Fundamentals M. T. Klein Outline 1. Elementary Steps 2. Alkyl Aromatics 3. Alkyl Naphthenics 4. Hydroaromatics 5. Conclusions Thermal Cracking Fundamentals 1 © Michael T. Klein et al. Cracking Fundamentals CATALYTIC: Carbenium Ion Stability Controls THERMAL: Free Radical Stability Controls Thermal Cracking Fundamentals 2 © Michael T. Klein et al. Thermal Cracking: Elementary Steps 1. Bond Fission R - R' R• + R'• Primary 2. Hydrogen Transfer Radical Allowed R• + R'H RH + R'• R' • 3. Scission R + • Can Continue Bond 4. Radical Recombination/Disproportionation R• + R'• R - R' Recombination R + O' Disproportion Kinetics - SSA Standard - Long Chains Thermal Cracking Fundamentals 3 - Scission to 1° Radical OK, Not Great © Michael T. Klein et al. Pyrolysis Reaction Families 1. Bond Fission: R 1 - R 2 R1• + R 2• log10 (A/s -1 ) = 16 1, E* = d° (bond strength) Compound d°/kcal mol -1 t1/2 400°C t1/2 750°C Ph-Ph 113.7 1.6 x 10 14 y 37y PhCH 2Ph 89.6 2.4 x 10 6y 2.3h PhCH 2-CH 2Ph 61.4 14h 7.8ms 73.9 19y 3.6s PhCH 2-CH 2CH 2Ph a half life for homolysis ThermalPoutsma, Cracking Fundamentals M. L. Energy Fuels, Vol. 4, No. 2, 1990, p. 1. 4 © Michael T. Klein et al. Pyrolysis Reaction Families 2. Hydrogen Abstraction (Transfer): R 1• + RH R1H + R• log10 (A/l mol -1 s -1 ) = ~8 E*/kcal mol -1 = ~12-20 log10 k400 = 2-5 Polanyi Relation E* = E* 0 - q 11.5 0.25 Thermal Cracking Fundamentals 5 © Michael T.