DMACC Chemical Hygiene Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COMBINED LIST of Particularly Hazardous Substances
COMBINED LIST of Particularly Hazardous Substances revised 2/4/2021 IARC list 1 are Carcinogenic to humans list compiled by Hector Acuna, UCSB IARC list Group 2A Probably carcinogenic to humans IARC list Group 2B Possibly carcinogenic to humans If any of the chemicals listed below are used in your research then complete a Standard Operating Procedure (SOP) for the product as described in the Chemical Hygiene Plan. Prop 65 known to cause cancer or reproductive toxicity Material(s) not on the list does not preclude one from completing an SOP. Other extremely toxic chemicals KNOWN Carcinogens from National Toxicology Program (NTP) or other high hazards will require the development of an SOP. Red= added in 2020 or status change Reasonably Anticipated NTP EPA Haz list COMBINED LIST of Particularly Hazardous Substances CAS Source from where the material is listed. 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide Acutely Toxic Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- Acutely Toxic 1-(2-Chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea (Methyl-CCNU) Prop 65 KNOWN Carcinogens NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) IARC list Group 2A Reasonably Anticipated NTP 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) (Lomustine) Prop 65 1-(o-Chlorophenyl)thiourea Acutely Toxic 1,1,1,2-Tetrachloroethane IARC list Group 2B 1,1,2,2-Tetrachloroethane Prop 65 IARC list Group 2B 1,1-Dichloro-2,2-bis(p -chloropheny)ethylene (DDE) Prop 65 1,1-Dichloroethane -

Technical/Application Article 02 Version 1.10 22Nd January 2018 WRH/FD
Technical/Application Article 02 Version 1.10 22nd January 2018 WRH/FD Ion Science PID Response Factors PID Response Photoionisation Detectors (PIDs) respond to a broad range of organic and a few inorganic gaseous and volatile chemicals (‘volatiles’). In order for PID to respond to a volatile, the photon energy of the lamp must be greater than its ionisation energy (IE). Ion Science PIDs are available with lamps emitting light of maximum energy of 10.0 eV, 10.6 eV, and 11.7 eV. This Technical Article lists the response factors (‘RF’s’) for over 900 volatiles with PID incorporating these lamps. The RF relates the sensitivity of PID to a volatile to the sensitivity to the standard calibration gas isobutylene. The higher the RF, the lower the sensitivity. Isobutylene as Reference Gas Ideally, the PID response to a chemical volatile would be calibrated by using a low concentration of the chemical in air. However, this is often not practical. Isobutylene is then used to calibrate PID, and a Response Factor (RF) used to convert the isobutylene calibrated measurement to a measurement of the target volatile: Concentration of target chemical = isobutylene calibrated measurement x RF For example, the RF of anisole is 0.59 with a 10.6 eV lamp. Therefore, a reading of 10 ppm using an isobutylene-calibrated unit would indicate: Concentration of anisole = 10 ppm x 0.59 = 5.9 ppm In Ion Science detectors, RFs are pre-programmed into a compound library and can be called up to make the PID read out in units of the chemical of interest. -

Chemical Hygiene Plan Manual
CHEMICAL HYGIENE PLAN AND HAZARDOUS MATERIALS SAFETY MANUAL FOR LABORATORIES This is the Chemical Hygiene Plan specific to the following areas: Laboratory name or room number(s): ___________________________________ Building: __________________________________________________________ Supervisor: _______________________________________________________ Department: _______________________________________________________ Telephone numbers 911 for Emergency and urgent consultation 48221 Police business line 46919 Fire Dept business line 46371 Radiological and Environmental Management Revisied on: Enter a revision date here. All laboratory chemical use areas must maintain a work-area specific Chemical Hygiene Plan which conforms to the requirements of the OSHA Laboraotry Standard 29 CFR 19190.1450. Purdue University laboratories may use this document as a starting point for creating their work area specific CHP. Minimally this cover page is to be edited for work area specificity (non-West Lafayette laboratories are to place their own emergency, fire, and police telephone numbers in the space above) AND appendix K must be completed. This instruction and information box should remain. This model CHP is version 2010A; updates are to be found at www.purdue.edu/rem This page intentionally blank. PURDUE CHEMICAL HYGIENE PLAN AWARENESS CERTIFICATION For CHP of: ______________________________ Professor, building, rooms The Occupational Safety and Health Administration (OSHA) requires that laboratory employees be made aware of the Chemical Hygiene Plan at their place of employment (29 CFR 1910.1450). The Purdue University Chemical Hygiene Plan and Hazardous Materials Safety Manual serves as the written Chemical Hygiene Plan (CHP) for laboratories using chemicals at Purdue University. The CHP is a regular, continuing effort, not a standby or short term activity. Departments, divisions, sections, or other work units engaged in laboratory work whose hazards are not sufficiently covered in this written manual must customize it by adding their own sections as appropriate (e.g. -

Biochemical and Pathological Effects of Methylazoxymethanol Acetate, a Potent Carcinogen1
[CANCER RESEARCH 30, 801-812, March 1970] Biochemical and Pathological Effects of Methylazoxymethanol Acetate, a Potent Carcinogen1 Morris S. Zedeck, Stephen S. Sternberg, Richard W. Poynter, and Jane McGowan Divisions of Pharmacology and Cytology, Sloan-Kettering Institute for Cancer Research, New York, New York 10021 SUMMARY vealed that the agent is cleaved by the intestinal flora which possess a 0-o-glucosidase, and that the free agly Methylazoxymethanol acetate is known to induce tu cone methylazoxymethanol is the active carcinogen (13- mors in rodents. Effects produced by a single nonlethal 15, 34). Matsumoto et al. (19) synthesized MAM acetate3 dose on nucleic acid and protein synthesis have been in (Chart 1), which is stable and not dependent upon vestigated in rat and mouse liver, small intestine, and ,0-glucosidase cleavage for activity. Single or only a few kidney, the 3 organs most susceptible to carcinogenesis. doses of cycasin, or of the aglycone methylazoxymetha The agent induced early inhibition of thymidine incor nol, are required to induce tumors in rats which are pri poration into DNA of rat tissues. Rat liver was most sen marily of the kidney, intestinal tract, and liver (11, 15). sitive to this effect and was the only organ to show marked Aqueous extracts of cycad nut have also been shown to inhibition of RNA and protein synthesis. In mice, liver induce tumors of liver and kidneys in mice (21). The was more sensitive than small intestine or kidney and ex present investigation was undertaken to study the acute hibited an inhibition of RNA and protein synthesis; there pathological and biochemical effects produced by MAM were no changes observed in small intestine or kidney. -

Chromatographic Separation of Alkaline Earth Metals Using Alpha-Hydroxyisobutyric Acid
AN ABSTRACT OF THE THESIS OF JOHN ARTHUR HAUSCHILD for the MASTER OF SCIENCE (Name) (Degree) in CHEMISTRY (ANALYTICAL) presented on (Major) Title: CHROMATOGRAPHIC SEPARATION OF ALKALINE EARTH METALS USING ALPHA-HYDROXYISOBUYRIC ACID Abstract approved: Redacted for Privacy Max B. Williams A systematic study of the elution of magnesium and calcium from Dowex 50 X 8 resin using a-hydroxyisobutyric acid (a-HIBA) at various pH values and concentrations, indicated that the difference in the equilibrium distribution coefficients of these two elements was large enough for a good separation.This fact was applied to develop a chromatographic procedure for the separationof milligram quantities of magnesium, calcium, strontium, and barium.After magnesium was eluted with 0. 22M a-HIBA at pH 4. 5, thethree remaining elements were eluted by varying the concentration and pH of a-HIBAduring the course of the elution (exponential gradient elution).After its respec- tive elution, each alkaline earth metal was directly determined by atomic absorption spectroscopy.Using this method, several success- ful analyses of synthetic samples (similar to the composition of sea water) were performed.Yield determinations of the alkaline earth metals from these analyses were consistently greater than 93%, with the overall average yield being 98%. Chromatographic Separation of Alkaline Earth Metals Using Alpha-Hydroxyisobutyric Acid by John Arthur Haus child A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master -

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -
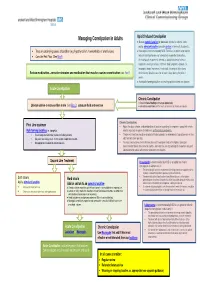
Managing Constipation in Adults
Managing Constipation in Adults Opio id Induced Constipation ● Use an osmotic laxative (or docusate which also softens stools and a stimulant laxative (consider dantron in terminally ill patients). • Treat any underlying causes of condition (e.g. hypothyroidism, haemorrhoids or anal fissures) ● Naloxegol is recommended by NICE TA345 as an option when opioid induced constipation has not adequately responded to laxatives. • Consider Red Flags (See Box A ) An inadequate response is defined as opioid-induced of at least moderate severity in at least 1of the 4 stool symptoms domain (i.e. incomplete bowel movement, hard stool, straining or false alarm) Review medication –consider stopping any medication that may be causing constipation (see Box B ) whilst taking1 laxative class for at least 4 days during the prior 2 weeks. ● Avoid bulk -forming l axatives for treating opioid induced constipation Acute Constipation Chronic Constipation ● Check for faecal loading and manage appropriately Lifestyle advise -to increase fibre in diet (see Box C ), adequate fluid and exercise ● Set realistic expectations for the results of treatment of chronic constipation. Chronic Constipation: First Line treatment • Adjust the dose, choice, and combination of laxative according to symptoms, speed with which Bulk forming laxatives i.e. ispaghula. relief is required, response to treatment, and individual preference. • Ensure adequate fluid intake (caution- frail elderly patients) • The dose of laxative should be gradually titrated upwards (or downwards) to produce one or two • May take several days to act- so not suitable if rapid relief required. soft, formed stools per day. • Not appropriate for opiod induced constipation. • If at least two laxatives (from different classes) have been tried at the highest tolerated recommended doses for at least 6 months, consider the use of prucalopride in women only and lubiprostone for adults with chronic idiopathic constipation. -

COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care
COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care In this issue: Glossary Page Chronic Constipation lasting longer than 3 months Introduction and background 1 constipation Drugs used in the management of Functional Chronic constipation without a known cause. Also known as primary 3 chronic constipation constipation constipation or idiopathic constipation Bulk-forming laxatives 4 Gastrocolic The occurrence of peristalsis following the entrance of food into the Osmotic laxatives 5 response empty stomach Stimulant laxatives 6 IBS Irritable Bowel Syndrome IBS-C Irritable Bowel Syndrome with Constipation Faecal softeners 7 Melanosis Dark brownish black pigmentation of the mucous membrane of the colon Peripheral opioid-receptor 7 coli due to the deposition of pigment in macrophages antagonists Myenteric Part of the enteric nervous system with an important role in regulating 5HT4 – receptor agonists 7 plexus gut motility Managing constipation in 8 NNT Number Needed to Treat pregnancy and breastfeeding OTC Over The Counter RCT Randomised Controlled Trial Secondary Constipation caused by a drug or medical condition. Secondary constipation constipation is also known as organic constipation SmPC Summary of Product Characteristics A twisting or looping of the bowel resulting in obstruction; can be life- Volvulus threatening Successful completion of the assessment questions at the end of this issue will provide you with 2 hours towards your CPD/CME requirements. Further copies of this and any other edition in the -

Fine Biocompatible Powders Synthesized from Calcium Lactate and Ammonium Sulfate
ceramics Article Fine Biocompatible Powders Synthesized from Calcium Lactate and Ammonium Sulfate Maksim Kaimonov 1,* , Tatiana Shatalova 1,2 , Yaroslav Filippov 1,3 and Tatiana Safronova 1,2 1 Department of Materials Science, Lomonosov Moscow State University, Building, 73, Leninskie Gory, 1, 119991 Moscow, Russia; [email protected] (T.S.); fi[email protected] (Y.F.); [email protected] (T.S.) 2 Department of Chemistry, Lomonosov Moscow State University, Building, 3, Leninskie Gory, 1, 119991 Moscow, Russia 3 Research Institute of Mechanics, Lomonosov Moscow State University, Michurinsky pr., 1, 119192 Moscow, Russia * Correspondence: [email protected]; Tel.: +7-952-889-11-43 Abstract: Fine biocompatible powders with different phase compositions were obtained from a 0.5 M solution of ammonium sulfate (NH4)2SO4 and calcium lactate Ca(C3H5O3)2. The powder ◦ after synthesis and drying at 40 C included calcium sulfate dehydrate CaSO4·2H2O and calcite ◦ CaCO3. The powder after heat treatment at 350 C included β-hemihydrate calcium sulfate β- CaSO4·0.5H2O, γ-anhydrite calcium sulfate γ-CaSO4 and calcite CaCO3. The phase composition of ◦ powder heat-treated at 600 C was presented as β-anhydrate calcium sulfate β-CaSO4 and calcite ◦ CaCO3. Increasing the temperature up to 800 C leads to the sintering of a calcium sulfate powder β β consisting of -anhydrite calcium sulfate -CaSO4 main phase and a tiny amount of calcium oxide CaO. The obtained fine biocompatible powders of calcium sulfate both after synthesis and after heat Citation: Kaimonov, M.; Shatalova, treatment at temperature not above 600 ◦C can be recommended as a filler for producing unique T.; Filippov, Y.; Safronova, T. -

Carbon Tetrachloride
CARBON TETRACHLORIDE Data were last reviewed in IARC (1979) and the compound was classified in IARC Monographs Supplement 7 (1987a). 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Nomenclature Chem. Abstr. Serv. Reg. No.: 56-23-5 Chem. Abstr. Name: Tetrachloromethane IUPAC Systematic Name: Carbon tetrachloride Synonyms: Benzinoform; carbona 1.1.2 Structural and molecular formulae and relative molecular mass Cl Cl CCl Cl CCl4 Relative molecular mass: 153.82 1.1.3 Chemical and physical properties of the pure substance (a) Description: Colourless, clear, nonflammable, liquid with a characteristic odour (Budavari, 1996) (b) Boiling-point: 76.8°C (Lide, 1997) (c) Melting-point: –23°C (Lide, 1997) (d) Solubility: Very slightly soluble in water (0.05% by volume); miscible with benzene, chloroform, diethyl ether, carbon disulfide and ethanol (Budavari, 1996) (e) Vapour pressure: 12 kPa at 20°C; relative vapour density (air = 1), 5.3 at the boiling-point (American Conference of Governmental Industrial Hygienists, 1991) (f) Conversion factor: mg/m3 = 6.3 × ppm 1.2 Production and use Production in the United States in 1991 was reported to be approximately 143 thousand tonnes (United States International Trade Commission, 1993). Information –401– 402 IARC MONOGRAPHS VOLUME 71 available in 1995 indicated that carbon tetrachloride was produced in 24 countries (Che- mical Information Services, 1995). Carbon tetrachloride is used in the synthesis of chlorinated organic compounds, including chlorofluorocarbon refrigerants. It is also used as an agricultural fumigant and as a solvent in the production of semiconductors, in the processing of fats, oils and rubber and in laboratory applications (Lewis, 1993; Kauppinen et al., 1998). -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials.