BRGMON-6 | Seton Lake Aquatic Productivity Monitoring
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

British Columbia Geological Survey Geological Fieldwork 1989
GEOLOGY AND MINERAL OCCURRENCES OF THE YALAKOM RIVER AREA* (920/1, 2, 92J/15, 16) By P. Schiarizza and R.G. Gaba, M. Coleman, Carleton University J.I. Garver, University of Washington and J.K. Glover, Consulting Geologist KEYWORDS:Regional mapping, Shulaps ophiolite, Bridge REGIONAL GEOLOGY River complex, Cadwallader Group Yalakom fault, Mission Ridge fault, Marshall Creek fault. The regional geologic setting of the Taseko-Bridge River projectarea is described by Glover et al. (1988a) and Schiarizza et al. (1989a). The distributicn and relatio~uhips of themajor tectonostratigraphic assemblages are !;urn- INTRODUCTION marized in Figures 1-6-1 ;and 1-6-2. The Yalakom River area covers about 700 square kilo- The Yalakom River area, comprisinl: the southwertem metres of mountainous terrain along the northeastern margin segment of the project area, encompasses the whole OF the of the Coast Mountains. It is centred 200 kilometres north of Shubdps ultramafic complex which is interpreted by hagel Vancouver and 35 kilometresnorthwest of Lillooet.Our (1979), Potter and Calon et a1.(19901 as a 1989 mapping provides more detailed coverageof the north- (1983, 1986) dismembered ophiolite. 'The areasouth and west (of the em and western ShulapsRange, partly mapped in 1987 Shulaps complex is underlain mainly by Cjceanic rocks cf the (Glover et al., 1988a, 1988b) and 1988 (Schiarizza et al., Permian(?)to Jurassic €!ridge Rivercomplex, and arc- 1989d, 1989b). and extends the mapping eastward to include derived volcanic and sedimentary rocksof the UpperTri %sic the eastem part of the ShulapsRange, the Yalakom and Cadwallader Group. These two assemhkgesare struclurally Bridge River valleys and the adjacent Camelsfoot Range. -

Community Risk Assessment
COMMUNITY RISK ASSESSMENT Squamish-Lillooet Regional District Abstract This Community Risk Assessment is a component of the SLRD Comprehensive Emergency Management Plan. A Community Risk Assessment is the foundation for any local authority emergency management program. It informs risk reduction strategies, emergency response and recovery plans, and other elements of the SLRD emergency program. Evaluating risks is a requirement mandated by the Local Authority Emergency Management Regulation. Section 2(1) of this regulation requires local authorities to prepare emergency plans that reflects their assessment of the relative risk of occurrence, and the potential impact, of emergencies or disasters on people and property. SLRD Emergency Program [email protected] Version: 1.0 Published: January, 2021 SLRD Community Risk Assessment SLRD Emergency Management Program Executive Summary This Community Risk Assessment (CRA) is a component of the Squamish-Lillooet Regional District (SLRD) Comprehensive Emergency Management Plan and presents a survey and analysis of known hazards, risks and related community vulnerabilities in the SLRD. The purpose of a CRA is to: • Consider all known hazards that may trigger a risk event and impact communities of the SLRD; • Identify what would trigger a risk event to occur; and • Determine what the potential impact would be if the risk event did occur. The results of the CRA inform risk reduction strategies, emergency response and recovery plans, and other elements of the SLRD emergency program. Evaluating risks is a requirement mandated by the Local Authority Emergency Management Regulation. Section 2(1) of this regulation requires local authorities to prepare emergency plans that reflect their assessment of the relative risk of occurrence, and the potential impact, of emergencies or disasters on people and property. -

Aesthetic Impact Informational Services, LLC Remote Viewing
Aesthetic Impact Informational Services, LLC Remote Viewing Educational Example Remote Viewing Target 130703 Long Freight Train – Canadian Pacific Railway, Seton Lake, British Columbia Coordinates: 130703 Blind Tasking: The target is a location. Describe the location. Online Discussion: https://www.youtube.com/watch?v=pHplxCMHmJc CRV Session Sketches, Summary & Topology Information contributed by Ronald Kuhn, Ohio, USA ----------- Seton Lake is a freshwater fjord draining east via the Seton River into the Fraser River at the town of Lillooet, British Columbia, about 22 km long and 243 m in elevation and 26.2 square kilometres in area.[1] Its depth is 1500 feet. The lake is natural in origin but was raised slightly as part of the Bridge River Power Project, the two main powerhouses of which are on the north shore of the upper end of the lake near Shalalth. At the uppermost end of the lake is the community of Seton Portage and the 1 mouth of the short Seton Portage River, which connects Anderson Lake on the farther side of the Portage to Seton Lake. Retrieved Mar. 1, 2015. http://en.wikipedia.org/wiki/Seton_Lake Image courtesy of Larry Bourne Sketch courtesy of Ronald Kuhn, CRV Intermediate Level Student The Bridge River hydroelectric complex consists of three dams and stores water for four generating stations. The system uses Bridge River water three times in succession to generate 492 megawatts, or 6 to 8 per cent of British Columbia's electrical supply. Hydroelectric development of the system began in 1927 and was completed in 1960. Its waters (Downton Reservoir) initially pass through the Lajoie Dam and powerhouse and are then diverted through tunnels and penstocks from Carpenter Reservoir to the two powerhouses on Seton Lake Reservoir. -

Seton Ridge Trail
Code: GC3QN9X Rails & Trails Written and Researched by Wayne Robinson Seton Ridge Trail Site Identification Nearest Community: Lillooet, B.C. Geocache Location: N 50°38.913' W 122°07.020' Ownership: Crown Land Accuracy: Photo: Wayne Robinson 5 meters Overall Difficulty: 3 Overall Terrain: 4.5 Access Information and Seton Ridge follows the height of the land with dizzyingly Restrictions: steep drops of nearly 1600 meters to either side. Seton From the Mile 0 cairn on Main Street follow Hwy 99 South on the Duffey Ridge is the eastern terminus of the Cayoosh Ranges of the Lake Road for 19.5 km and turn right Coast Mountains of British Columbia. To the north of the on Seton Ridge Forstery Service Road. trail is Seton Lake and to the south, the Cayoosh Creek Cross the bridge over Cayoosh Creek, valley. Cayoosh Creek originates just west of Duffy Lake and continue on about 6 km to flat area on the left. Trail is adequately marked in Cayoosh Pass, close to Lillooet Lake. Seton Lake is with flagging tape. 4x4 with high classified as a freshwater fjord that drains to the east into clearance. Cayoosh Creek which is referred to as the Seton River in the BC Freshwater Fishing Regulations. Seton Lake’s Parking Advice: actual depth is not entirely known but is known to exceed Park in pull out. Trail starts to your left. 500 meters. Although it is called a lake, Seton is a reservoir; the eastern end was dammed as a part of the Bridge River Power complex that was completed in 1960. -

Seton River Habitat and Fish Monitoring | Year 7
Bridge River Project Water Use Plan Seton River Habitat and Fish Monitoring Implementation Year 7 Reference: BRGMON-09 Study Period: January to December 2019 Jennifer Buchanan, Daniel Ramos-Espinoza, Annika Putt, Katrina Cook, Stephanie Lingard Instream Fisheries Research Inc. Splitrock Environmental Unit 115 – 2323 Boundary Rd., 1119 Hwy 99 South Vancouver, BC. PO Box 798 V5M 4V8 Lillooet, BC T: 1 (604) 428 – 8819 V0K 1V0 August 31, 2020 Bridge-Seton Water Use Plan Implementation Year 7 (2019): Seton River Habitat and Fish Monitoring Reference: BRGMON-9 Jennifer Buchanan, Daniel Ramos-Espinoza, Annika Putt, Katrina Cook, Stephanie Lingard Prepared for: Splitrock Environmental 1119 Hwy 99 South PO Box 798 Lillooet, BC V0K 1V0 Prepared by: InStream Fisheries Research Inc. 115 – 2323 Boundary Road Vancouver, BC V5M 4V8 Bridge-Seton Water Use Plan BRGMON-9: Seton River Habitat and Fish Monitoring August 31, 2020 Executive Summary The overall objective of the BRGMON-9 program is to monitor responses of fish habitat and fish populations in the Seton River to the Seton Dam hydrograph. Currently in year seven of ten, this monitoring program was developed to address a series of management questions (MQ) that aim to: 1) better understand the basic biological characteristics of the rearing and spawning fish populations in Seton River, 2) determine how the Seton River hydrograph influences the hydraulic condition of juvenile fish rearing habitats and fish populations, 3) evaluate potential risks of salmon and steelhead redds dewatering due to changes in the Seton River hydrograph, 4) assess how the Seton River hydrograph influences the availability of gravel suitable for spawning, and 5) estimate the effects of discharge from the Seton Generating Station (SGS) on fish habitat in the Fraser River. -

British Columbia Coastal Range and the Chilkotins
BRITISH COLUMBIA COASTAL RANGE AND THE CHILKOTINS The Coast Mountains of British Columbia are remote with limited accessibility by float plane, helicopter or boating up its deep inlets along the coast and hiking in. The mountains along British Columbia and SE Alaska intermix with the sea in a complex maze of fjords, with thousands of islands. It is a true wilderness where not exploited by logging and salmon farming pens. But there are some areas accessible from roads that can be explored, including west of Lillooet, the Chilcotins, and the Garibaldi Range. The Coast Mountains extend approximately 1,600 kilometres (1,000 mi) long from the southeastern boundaries are surrounded by the Fraser River and the Interior Plateau while its far northwestern edge is delimited by the Kelsall and Tatshenshini Rivers at the north end of the Alaska Panhandle, beyond which are the Saint Elias Mountains. The western mountain slopes are covered by dense temperate rainforest with heavily glaciated peaks and icefields that include Mt Waddington and Mt Silverthrone. Mount Waddington is the highest mountain of the Coast Mountains and the highest that lies entirely within British Columbia, located northeast of the head of Knight Inlet with an elevation of 4,019 metres (13,186 ft). The range along its eastern flanks tapers to the dry Interior Plateau and the boreal forests of the southern Chilkotins north to the Spatsizi Plateau Wilderness Provincial Park. The mountain range's name derives from its proximity to the sea coast, and it is often referred to as the Coast Range. The range includes volcanic and non-volcanic mountains and the extensive ice fields of the Pacific and Boundary Ranges, and the northern end of the volcanic system known as the Cascade Volcanoes. -

Informing the Survival of Fraser Sockeye Returning in 2020 Through Life Cycle Observations
State of the Salmon: Informing the survival of Fraser Sockeye returning in 2020 through life cycle observations Bronwyn L. MacDonald, Sue C.H. Grant, Niki Wilson, David A. Patterson, Kendra A. Robinson, Jennifer L. Boldt, Jackie King, Erika Anderson, Scott Decker, Brian Leaf, Lucas Pon, Yi Xu, Brooke Davis, and Dan T. Selbie Fisheries and Oceans Canada Science Branch, Pacific Region Pacific Biological Station 3190 Hammond Bay Road Nanaimo, British Columbia V9T 6N7 2020 Canadian Technical Report of Fisheries and Aquatic Sciences 3398 1 Canadian Technical Report of Fisheries and Aquatic Sciences Technical reports contain scientific and technical information that contributes to existing knowledge but which is not normally appropriate for primary literature. Technical reports are directed primarily toward a worldwide audience and have an international distribution. No restriction is placed on subject matter and the series reflects the broad interests and policies of Fisheries and Oceans Canada, namely, fisheries and aquatic sciences. Technical reports may be cited as full publications. The correct citation appears above the abstract of each report. Each report is abstracted in the data base Aquatic Sciences and Fisheries Abstracts. Technical reports are produced regionally but are numbered nationally. Requests for individual reports will be filled by the issuing establishment listed on the front cover and title page. Numbers 1-456 in this series were issued as Technical Reports of the Fisheries Research Board of Canada. Numbers 457-714 were issued as Department of the Environment, Fisheries and Marine Service, Research and Development Directorate Technical Reports. Numbers 715-924 were issued as Department of Fisheries and Environment, Fisheries and Marine Service Technical Reports. -

Mule Deer Buck Migrations and Habitat Use in the Bridge River, British Columbia: Preliminary Results (FWCP Project # 12.W.BRG.03)
Mule Deer Buck Migrations and Habitat Use in the Bridge River, British Columbia: Preliminary Results (FWCP Project # 12.W.BRG.03) PREPARED BY: Chris Procter, R.P.Bio Francis Iredale, R.P.Bio Ministry of Forests, Lands & Natural Resource Operations Fish & Wildlife Kamloops, BC PREPARED FOR: Fish & Wildlife Compensation Program-Coastal MARCH 31, 2013 EXECUTIVE SUMMARY In recent years, both the Ministry of Forests, Lands and Natural Resource Operations (MFLNRO) and the St’at’imc First Nation have become concerned with the status of the mule deer population west of the Fraser in southcentral British Columbia. These concerns provided the impetus for the recently completed two years of research on the female components of the deer population in the St’at’imc territory. This project seeks to build and expand on that data set by investigating habitat use and migration ecology of mule deer bucks in the area to provide further information on this population that can be applied to deer management. The primary purpose of this report is to report preliminary results from the first sampling session (i.e., first 2 years of the project). During April and May 2011, 9 mule deer bucks were captured and collared in the study area through free-range chemical immobilization. Collars were retrieved during April and May 2012 and data was downloaded for analysis. Overall, 78% of bucks migrated to distant summer ranges entirely separated from spring/winter ranges. Migrations were generally characterized as relatively straight in a westerly direction along the south aspect slopes on the north side of Carpenter Lake with use of interspersed transitional ranges along the way. -
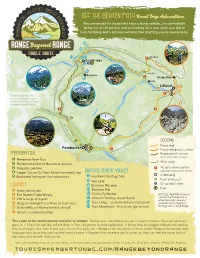
Download Range Beyond Range Circle Route
off the Beaten Path Road Trip Adventure... Recommended for those who have a sturdy vehicle, are comfortable being out of cell service, and are looking for a new circle tour that is both humbling and a bit more extreme than anything you’ve experienced. 19 L ● n to h g Ab u out a 1 y h T o Lillooet Pio u 18 . / neer r ● Rd Rd 4 4 ke 0 5 ter La B pen r m ar i C C i d n arp g ute ke en e s a t d L er R r Yalakom ●13 n ●17 L iv u ●16 a i v e* ●! G k Carp e ent e B er r r Gold Bridge L id D ak g ow e V e n R R L a iv to g d e n i l l i r L ● . l l ● 12 e Road a o y / k ● o / L e e il t L l i o P ll o i o e o o t n et Pi e P on e io e F r n e r e R e r a r R o R s d d a 40 e d 14 4 r ● 0 Terzhagi Dam R i v ad e Ro r * rley Bralorne e Hu v st i Ea ●! r Bridge River d r 15 e ● b M o t o s c Tsal’alh h a O r – S R s e e d n t o . u s u n J L a l o a a n k Lillooet P o e ●7 h s 11 a ● y e 6 s ● 2 e - l R Seton Lake Lookout S e i 10 t F ● r k ● er a u u iv R L o y n ! 99 9 H e ● ● F l y g b r o w ● r u s H a / s *Road conditions may A H r ad e e Ro r e Lak d y R fe n f iv vary, resulting in longer u e A D r travel times. -

The Reproductive Biology of Steelhead (Oncorhynchus Mykiss) in the Bridge and Seton Rivers, As Determined by Radio Telemetry 1996/97 and 1998/99
The Reproductive Biology of Steelhead (Oncorhynchus mykiss) in the Bridge and Seton Rivers, As Determined by Radio Telemetry 1996/97 and 1998/99 Prepared for: The Ministry of Environment, Lands & Parks Fisheries Branch, Southern Interior Region 1259 Dalhousie Dr. Kamloops, BC V2C 5Z5 Prepared by: Stacy Webb, Robert Bison, Al Caverly and Jim Renn Abstract The 1996/97 and 1998/99 studies of the spawning migrations of Bridge and Seton River steelhead were part of a larger study investigating the migration behaviour and stock composition of interior Fraser River steelhead. Steelhead were radio-tagged in the fall of 1996 and 1998 in the Lower Fraser River and in the winter/spring of 1997 and 1999 in the Middle Fraser River. Tagging effort was concentrated at the Seton/Fraser River confluence during the winter/spring captures, specifically to study Bridge and Seton River steelhead. A total of 15 steelhead were tracked during the 1997 spawning season and 18 steelhead were tracked during the 1999 spawning season in the Bridge and Seton watersheds. Immigration into the Seton and Bridge Rivers started around the middle of April and finished during the second week of May. Immigration into the Bridge and Seton Rivers in 1999 occurred primarily during the last two weeks of April. Spawning in the Bridge and Seton watersheds in 1997 started during the second week of May and ended around the middle of June. Spawning in the Bridge and Seton watersheds in 1999 occurred a little earlier, starting during the second week of April and finishing during the first week of June. -

BRGMON-8 | Seton Lake Resident Fish Habitat and Population
Bridge River Project Water Use Plan Seton Lake Resident Fish Habitat and Population Monitoring Implementation Year 3 Reference: BRGMON-8 BRGMON-8 Seton Lake Resident Fish Habitat and Population Monitoring, Year 3 (2015) Results Study Period: April 1 2015 to March 31 2016 Jeff Sneep and St’at’imc Eco-Resources February 6, 2018 BRGMON-8 Seton Lake Resident Fish Habitat and Population Monitoring, Year 3 (2015) Results Prepared for: St’at’imc Eco-Resources Prepared by: Jeff Sneep Lillooet, BC Canada File no. BRGMON-8 February 2018 Seton Lake Resident Fish Habitat and Population Monitoring Year 3 (2015) Executive Summary Data collection for Year 3 of this proposed 10-year study was completed in 2015. Results for Years 1 and 2 of this program are provided in the previous data report produced for this program in 2015 (Sneep 2015). Where relevant, comparisons across monitoring years for this program have been included in this report. A full synthesis of all results will be conducted following the final year of data collection which is scheduled for 2022. The primary objective of this monitoring program is to “collect better information on the relative abundance, life history and habitat use of resident fish populations in Seton Lake” (BC Hydro 2012). Field studies for the Seton Lake Resident Fish Habitat and Population Monitoring Program (BRGMON-8) were conducted in Seton Lake, as well as Anderson Lake for the first time this year. Data collection in Anderson Lake was included to provide context and comparison for the Seton Lake results. The two lakes are comparably sized, located within the same watershed, and have similar natural inflows; however, Seton Lake is impacted by the diversion from Carpenter Reservoir whereas Anderson Lake is not. -

BRGMON-9 | Seton River Habitat and Fish Monitor
Bridge-Seton Water Use Plan Seton River Habitat and Fish Monitor Implementation Years 1 and 2 Reference: BRGMON-9 Study Period: March 1 to December 31, 2014 Daniel Ramos-Espinoza, Douglas C Braun & Don McCubbing InStream Fisheries Research Inc. 1698 Platt Crescent, North Vancouver, BC. V6J 1Y1 January 2015 Bridge-Seton Water Use Plan Seton River Habitat and Fish Monitor: BRGMON-9 January, 2015 Seton River Habitat and Fish Monitor 2014 InStream Fisheries Research Inc. Page i Bridge-Seton Water Use Plan Seton River Habitat and Fish Monitor: BRGMON-9 January, 2015 Executive Summary The objective of this monitoring program is to monitor the response of fish habitat and fish populations to variations in Seton Dam flow operations. This monitor combines old and new approaches to better understand the status of the Seton River fish populations and how different life histories may be affected by Seton Dam operations. The data collected on juvenile and adult fish populations will, over time, allow us to identify trends and patterns that will enable us to make inferences about the effect of flow on habitat, species abundance and diversity. In 2014, we collected data through habitat (depth, velocity) surveys, which allowed us to quantify useable habitat for Rainbow Trout, Coho and Chinook juveniles in the Seton River. Repeating the surveys at established sites enabled us to monitor the effects of flows on each habitat type. In 2014, habitat surveys were completed at four different discharges: 12, 15, 25 and 27 m3/s. Overall, it appears that as flows increase, useable habitat decreases. However, this change is not consistent between habitat types and species.