Mbarara University of Science and Technology (MUST) – University of California, San Francisco Collaboration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Informal Support for People with Alzheimer's Disease and Related D
Informal Support for People With Alzheimer’s Disease and Related Dementias in Rural Uganda: A Qualitative Study Pia Ngoma Nankinga ( [email protected] ) Mbarara University of Science and Technology Samuel Maling Maling Mbarara University of Science and Technology Zeina Chemali Havard Medical School Edith K Wakida Mbarara University of Science and Technology Celestino Obua Mbarara University of Science and Technology Elialilia S Okello Makerere University Research Keywords: Informal support, dementia and rural communities Posted Date: December 17th, 2019 DOI: https://doi.org/10.21203/rs.2.19063/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/16 Abstract Background: The generation of people getting older has become a public health concern worldwide. People aged 65 and above are the most at risk for Alzheimer’s disease which is associated with physical and behavioral changes. This nurtures informal support needs for people living with dementia where their families together with other community members are the core providers of day to day care for them in the rural setting. Despite global concern around this issue, information is still lacking on informal support delivered to these people with dementia. Objective: Our study aimed at establishing the nature of informal support provided for people with dementia (PWDs) and its perceived usefulness in rural communities in South Western Uganda. Methods: This was a qualitative study that adopted a descriptive design and conducted among 22 caregivers and 8 opinion leaders in rural communities of Kabale, Mbarara and Ibanda districts in South Western Uganda. The study included dementia caregivers who had been in that role for a period of at least six months and opinion leaders in the community. -

Bishop Stuart University P.O
BISHOP STUART UNIVERSITY P.O. Box 9 Mbarara Uganda. Tel: +256-4854-22970 Email: [email protected], [email protected] Kampala Liason Offi ce: St. Francis Community Centre Phase II building, 2nd Floor, Room 1 Makerere University. Email: [email protected] Tel: +256-773-724-003 Website: www.bsu.ac.ug INTRODUCTION Medical Services: The University has been blessed with a clinic • Bachelor of Animal Health and Producti on (BAHP)* • Bachelor of Banking and Investment Management (BBIM) Under the guidance of lecturers, the students of the faculty will Bishop Stuart University is a not-for-profi t Chartered educati onal which is manned by well trained nurses. For referrals, the pati ents • Bachelor of Sports Science (BSS) • Bachelor of Project Planning and Management (BPPM)* be conducti ng clinics to assist people with various legal problems, insti tuti on established by Ankole Diocese of the Province of the are referred to Ruharo Mission Hospital with which the university • Diploma in Midwifery (DMW) • Bachelor of Procurement and Supply Chain Management such as accessing justi ce, issues if domesti c violence, matt ers of has a partnership/health scheme. Anglican Church of Uganda to provide Christi an based higher • Advanced Certi fi cate in Appropriate and Sustainable (BPSCM)* succession. They will be writi ng to sensiti ze communiti es about Technologies (ACAST) • Bachelor of Community Psychology (BCP)* their rights, such as the right to a clean environment, the right to Educati on, training and research for the expansion of God’s Students Clubs: Many clubs and associati ons are progressively The university got an opportunity of sending its students of • Bachelor of Records Management and Informati on Science educati on, the right to health and the right to shelter, land rights kingdom, human Knowledge and bett erment of society. -

BETTER GROWTH, BETTER CITIES Achieving Uganda’S Development Ambition
BETTER GROWTH, BETTER CITIES Achieving Uganda’s Development Ambition A paper by the Government of Uganda and the New Climate Economy Partnership November 2016 THE REPUBLIC OF UGANDA THE REPUBLIC OF UGANDA About this paper The analysis in this paper was produced for the New Climate Partnership in Uganda research project, culminating in the report, Achieving Uganda’s Development Ambition: The Economic Impact of Green Growth – An Agenda for Action. This National Urban Transition paper is published as a supporting working paper and provides a fuller elaboration of the urbanisation elements in the broader report. Partners Achieving Uganda’s Development Ambition: The Economic Impact of Green Growth – An Agenda for Action was jointly prepared by the Government of Uganda through the Ministry of Finance, Planning and Economic Development (MFPED), the Ugandan Economic Policy Research Centre (EPRC) Uganda, the Global Green Growth Institute (GGGI), the New Climate Economy (NCE), and the Coalition for Urban Transitions (an NCE Special Initiative). Ministry of Finance, Planning and Economic Development Plot 2/12 Apollo Kaggwa Road P.O.Box 8147 Kampala, Uganda +256-414-707000 COALITION FOR URBAN TRANSITIONS A New Climate Economy Special Initiative Acknowledgements The project team members were Russell Bishop, Nick Godfrey, Annie Lefebure, Filippo Rodriguez and Rachel Waddell (NCE); Madina Guloba (EPRC); Maris Wanyera, Albert Musisi and Andrew Masaba (MPFED); and Samson Akankiza, Jahan-zeb Chowdhury, Peter Okubal and John Walugembe (GGGI). The technical -

Facilitation of an IT Training Centre in Uganda
Justine Magambo 13.06.2010 Proposal for Facilitation of an IT Training Centre in Uganda Past Performance In 2004, the education-africa project was initiated at the University of Cologne with the major goal of facilitating ICT use in teacher education. Meanwhile, the University of Cologne has signed Memorandums of Understanding with Kenyatta University, Kyambogo University and Mbarara University of Science and Technology. In 2008 Linuxola in cooperation with the Department of Educational Psychology at the University of Cologne formed a partnership to provide three African universities with computer hardware and software. The major aim was to support teaching staff and student teachers in these universities in developing ICT skills both for general use and for teaching. This concept was proposed in response to the study carried out by Justine Magambo1 in 2007 which explored the use ICT in teacher education in universities in sub-Sahara Africa. Objectives and activities Within this framework, Linuxola donated 20 computers to each of the three universities: Kenyatta University (Kenya), Kyambogo University and Mbarara University of Science and Technology in Uganda. Linuxola was in charge of fundraising for the computers, while the University of Cologne financed shipping. The African universities were to ensure space or rooms for the computers as well as identify IT focal points. Justine Magambo, who coordinated and managed the project and the partnership, visited all three universities to appraise their preparedness for the computer consignment. Her report showed that all three universities had appointed IT focal points and set up computer rooms. In February 2010 the computers for Mbarara and Kyambogo were delivered and cleared by Mbarara University as agreed by both university officials. -

Uganda Country Strategy Paper 2017-2021
AFRICAN DEVELOPMENT BANK GROUP UGANDA COUNTRY STRATEGY PAPER 2017-2021 RDGE/COUG June 2017 TABLE OF CONTENTS EXECUTIVE SUMMARY ................................................................................................................. iii I. INTRODUCTION ............................................................................................................................. 1 II. THE COUNTRY CONTEXT ......................................................................................................... 1 2.1 Political Context ......................................................................................................................................... 1 2.2 Economic Context ...................................................................................................................................... 2 2.3 Social development and Cross-cutting Issues……………………………………………………………….. .............................................. 5 III. STRATEGIC OPTIONS, PORTFOLIO PERFORMANCE AND LESSONS ........................ 7 3.1 Country Strategic Framework .................................................................................................................. 7 3.2 Aid Coordination and Harmonization ...................................................................................................... 8 3.3 Country Challenges & Weaknesses and Opportunities and Strengths ................................................. 8 3.5 Key Findings of the CSP 2011-16 Country Portfolio Performance Review (CPPR) ......................... -

A Review of Land Tenure and Land Use Planning in the Six Kagera TAMP Districts in Uganda
A Review of Land Tenure and Land use Planning in the six Kagera TAMP Districts in Uganda BdBernard BhBashaas ha School of Agricultural Sciences, Makerere University. Outline of Presentation Background Objective and Methodology Research Questions PliiPreliminary Fi Fidindings Land tenure structure Background KLKey Laws and regu lati ons rel ati ng t o th e ownership, management and transfer of land and re ltdlated resources i n Ug Uand a. The Constitution • The Land Act (1998), • The Land regulations of 2004 • The Physical planning Act of 2010. This act specifically provides for the design and implementation of land use plans. Background cont’d Other Relevant Laws: • The land acquisition Act, • The Water Act and associated water resources regulati on s, • The National Forestry and tree planting Act (2003), • The National Environment Regulations (2001), • The mortgage Act, • the registration of titles Act and the rent restriction Act. Objective & Methodology Object ive To achieve a deeper understanding of the land tenure structure andld land use p lanni ng i n th e six Kagera TAMP districts Highlight the constraints and identify the opportun ities f or pri ority it acti on. Methodology A combination of literature review and key informant interviews with district level staff. Research Questions What is the current land tenure structure and its distribution in Uganda and in the six districts of the Kagera TAMP project? What are the strategies and plans in place to improve the land tenure structure in Uganda and in the six Kagera TAMP project districts, in particular? Do land use plans exist at the National level and in the six Kagera TAMP project districts? HdhiildHow do the existing land use pl ans compare (i n content, design process (approach) and coordination arrangements) with best practices. -

MBARARA CITY: Settlement Profiles
MBARARA MUNICIPALITY 2010 SLUM PROFILE PRODUCED BY ‘Joining hands with the Urban poor’ & UGANDA SLUM DWELLERS FEDERATION FORE WORD ACTogether Uganda, Uganda Slum Dwellers Federation in partnership with Mbarara Municipal council and Ministry of Lands Housing and Urban Development with support from SDI and Cities Alliance, found it necessary that they collect this information so that the challenges of the urban poor living in these slum settlements can be bought to the fore-front in planning and development of Mbarara Municipality. Our purpose of putting together these profiles is to provide an inner understanding and most uncomfortable truth about the status of the urban slum settlements in Mbarara. The report comprises of a list of slum settlements in Mbarara Municipality and carries the most standardized detail of the informal settlements as provided by slum dwellers them selves. It‟s paramount to note that the variables were not derived from professionals, academicians or technical people but rather the slum dwellers themselves who most understand the slum conditions simply because it‟s where they live and spend their time. We also found it very necessary to collect the past and present histories of each slum because with experience gained from working in slums, the history of each area has a significant role to play in its upgrading and development. Mbarara Municipal council has put in place necessary legal and regulatory mechanisms to safe guard the rights of people living in slum settlements and ensure that harmony, cohesion and inclusive development is given a priority. There fore it recognizes the presence, potentials and partnerships of people living in slums. -

Undergraduate Private Admissions 2020/2021 Academic Year
MBARARA UNIVERSITY OF SCIENCE AND TECHNOLOGY OFFICE OF THE ACADEMIC REGISTRAR P.O. Box 1410, MBARARA-UGANDA Telephone: +256-485-660584, +256-414-668971 Email: [email protected], [email protected] Web: www.must.ac.ug UNDERGRADUATE PRIVATE ADMISSIONS 2020/2021 ACADEMIC YEAR The following have been admitted to the different programmes as below for the 2020/2021 academic year. Admission letters shall be sent by email to applicants who have paid a NON-REFUNDABLE TUITION FEES DEPOSIT of Shs. 50,000=. Visit www.must.ac.ug for instructions on how to pay or contact us by email [email protected] or WhatsApp us on +256-786-706490. BACHELOR OF SCIENCE IN COMPUTER ENGINEERING SN NAME GENDER NATIONALITY DISTRICT ALEVEL_INDEX YEAR WEIGHT 1 BATAMYE ABDUL M Ugandan BUIKWE U1609/635 2019 47.1 2 BONGO JOSHUA M Ugandan APAC U2060/581 2019 44.2 3 KIA JANET F Ugandan ALEBTONG U1923/610 2019 43.7 4 NSHEKANABO MARIUS M Ugandan SHEEMA U1063/563 2019 41.3 5 BINTO NAOMI F Ugandan MUKONO U2583/568 2019 40.7 6 BWAMBALE ROBERT SEMAKULA M Ugandan KASESE U3231/514 2019 31.5 7 MUTEBI JONATHAN M Ugandan WAKISO U0053/823 2019 31.2 8 ARINAITWE JULIUS M Ugandan MBARARA U1495/554 2017 31.1 9 ATWIINE SAGIUS M Ugandan NTUNGAMO U0946/572 2019 28.0 10 KISAKYE JULIUS M Ugandan IGANGA U0027/564 2019 27.6 11 MUKWATANISE ALBERT M Ugandan ISINGIRO U0334/692 2019 27.6 12 MATEGE DERICK M Ugandan KAMULI U2877/614 2012 27.1 13 MUHUMUZA JOSEPH M Ugandan KISORO U0080/566 2019 25.2 14 MWEBESA TREVOR M Ugandan NTUNGAMO U0053/827 2019 25.2 15 KAANYI JANE PATIENCE F Ugandan KIBUKU U0065/586 -

A Livelihood Intervention to Improve Economic and Psychosocial Well-Being in Rural Uganda: Longitudinal Pilot Study
Portland State University PDXScholar OHSU-PSU School of Public Health Faculty Publications and Presentations OHSU-PSU School of Public Health 9-2016 A Livelihood Intervention to Improve Economic and Psychosocial Well-Being in Rural Uganda: Longitudinal Pilot Study Bernard Kakuhikire Mbarara University of Science and Technology Diego Suquillo Harvard College Elly Atuhumuza Infectious Diseases Research Collaboration, Kampala, Uganda Rumbidzai Mushavi Harvard Medical School Jessica M. Perkins Massachusetts General Hospital Follow this and additional works at: https://pdxscholar.library.pdx.edu/sph_facpub Part of the Health Services Research Commons, International Public Health Commons, and the Public HealthSee next Education page for andadditional Promotion authors Commons Let us know how access to this document benefits ou.y Citation Details Bernard Kakuhikire, Diego Suquillo, Elly Atuhumuza, Rumbidzai Mushavi, Jessica M. Perkins, Atheendar S. Venkataramani, Sheri D. Weiser, David R. Bangsberg & Alexander C. Tsai (2016) A livelihood intervention to improve economic and psychosocial well-being in rural Uganda: Longitudinal pilot study, SAHARA-J: Journal of Social Aspects of HIV/AIDS, 13:1, 162-169. This Article is brought to you for free and open access. It has been accepted for inclusion in OHSU-PSU School of Public Health Faculty Publications and Presentations by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. Authors Bernard Kakuhikire, Diego Suquillo, Elly Atuhumuza, Rumbidzai Mushavi, Jessica M. Perkins, Atheendar S. Venkataramani, Sheri D. Weiser, David Bangsberg, and Alexander C. Tsai This article is available at PDXScholar: https://pdxscholar.library.pdx.edu/sph_facpub/14 Original Article A livelihood intervention to improve economic and psychosocial well-being in rural Uganda: Longitudinal pilot study a b c d e Bernard Kakuhikire , Diego Suquillo , Elly Atuhumuza , Rumbidzai Mushavi , Jessica M. -

Licensed-Money-Remitters-As-At-15
OPERATIONAL MONEY REMITTANCE OUTLETS AS AT MARCH, 2016 NAME OF MONEY REMITTER ADDRESS/PHYSICAL LOCATION 1 ACCESS P.O.Box 27632 Kampala, Plot No.1744 Muyenga Road, Kabalagala, Kampala 2 ACCESS P.O.Box 27632 Kampala, Shop No.12 Krish Mall, Old Portbell Road, Bugolobi, Kampala. 3 AMAL P.O.Box 10363 Kampala, Plot No.18 Ben Kiwanuka, Kampala. 4 AMAL P.O.Box 10363 Kampala, Plot No.1 Central Lane, Arua 5 B. M. P.O. Box 7258 Kampala , Plot No. 8/10 Kampala Road, Uganda House, Kampala 6 BAKAAL P.O.Box 10161 Kampala, Plot 82 Ben Kiwanuka Street, Tropical Complex, Kampala. 7 BAKAAL P.O.Box 10161 Kampala, Plot 11 Malinga Road, Mengo Kisenyi, Kampala. 8 BESO EXPRESS P.O.Box 72911, Kampala, Plot No. 52 Kampala Road, King Fahd Plaza, First Floor, Shop No. S.9, Kampala. 9 BEST P.O. Box 22701 Kampala , Plot No. 25A&B William Street, Trade Link Building, Level 2, Kampala 10 BEST P.O Box 22701 Kampala, Shop No. D/G-03, Plot No. 40/41, Mukwano Arcade 11 BEST RATES P.O.Box 31448, Kampala , Capital Shoppers Shopping Centre, Shop 1 Ntinda Road, Kampala. 12 BIASHARA P.O.Box 3011 Kampala , Plot 01, johnstone street, Kampala. Shop No. 08/09 Kalungi Commercial Plaza 13 BICCO P.O Box 3307 Kampala, Plot No.01 Colville Street 14 BICCO P.O Box 3307 Kampala, Buganda Road, Plot 13, Mukwano Mall 15 BUDDU P.O.Box 23442 Kampalak, Plot No. 22A, Shop No.L3-662/3, Gaza land Plaza, William Street, Kampala 16 CAPITAL P.O. -
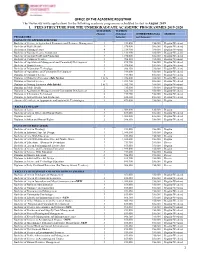
1 Office of the Academic Registrar 1. Fees Structure
OFFICE OF THE ACADEMIC REGISTRAR The University invite applications for the following academic programmes scheduled to start in August 2019 1. FEES STRUCTURE FOR THE UNDERGRADUATE ACADEMIC PROGRAMMES 2019-2020 DURATION TUITION (Years) (Ush) per OTHER FEES (Ush) SESSION PROGRAMES Semester per Semester FACULTY OF APPLIED SCIENCES Bachelor of Science in Agricultural Economics and Resource Management 3 919,600 356,000 Regular/Weekend Bachelor of Public Health 3 1,078,000 386,000 Regular/Weekend Bachelor of Nursing Science 4 1,318,900 386,000 Regular/Weekend Bachelor of Nursing Science Completion 3 1,318,900 386,000 Regular/Weekend Bachelor of Animal Health and Production 3 1,078,000 356,000 Regular/Weekend Bachelor of Computer Science 3 986,150 356,000 Regular/Weekend Bachelor of Agribusiness Management and Community Development 3 892,900 356,000 Regular/Weekend Bachelor of Sports Science 3 1,078,000 356,000 Regular/Weekend Bachelor of Information Technology 3 986,150 356,000 Regular/Weekend Bachelor of Agriculture and Community Development 3 919,600 356,000 Regular/Weekend Diploma in Computer Science 2 719,950 356,000 Regular/Weekend Diploma in Midwifery Extension (July Intake) 1 & ½ 1,056,000 386,000 Regular/Weekend Diploma in Nursing Science 2 1,052,700 386,000 Regular/Weekend Diploma in Nursing Extension (July Intake) 1 & ½ 1,052,700 386,000 Regular/Weekend Diploma in Public Health 2 715,000 356,000 Regular/Weekend Diploma in Agribusiness Management and Community Development 2 626,780 356,000 Regular/Weekend Diploma in Information Technology -

The Uganda Gazette Published
1721 The Published b\ Uganda Gazette Authoritx Vol. CXIII No. 58 2nd October, 2020 Price: Shs. 5,000 CONTENTS Page General Notice No, 1217 of 2020. Ihe Marriage Act Notices ... ... 1721 THE MARRIAGE AC T (Cap. 251 Revised Edition, 2000| I he Commissioners lor Oaths (Advocates) NOTICE Act -Notice ... ............... ■ 1721 /Under Section 5 of the Act/ I he Advocates Act—Notices ... ... 1722 PLACE EOR CELEBRATION OE MARRIAGE 'Hie Companies Act— Notices ... ... 1722-1723 Registry No: 1201000(1027704-00470 I he Mining Act - Notices ............... ... 1723-172-1 IN EXERCISE of the powers conferred upon me by Section Ihe Trademarks Act Registration of Applications 1724-1739 5 of the Marriage Act, I hereby licence the place of Public Worship mentioned in the Schedule hereto to be a place for z\dx •enisements ... ............... 1739-1800 celebration of marriages. SCHEDULE Church — Live Christian Centre Mission Church SUPPLEMENT Denomination — Pentecostal Si tuition Ins/ntnienf Village •— Kabanyoro Parish — Gayaza No. Ill - Ihe Diplomatic Privileges (Extension to Sub-County — Kasangati Town Council Prescribed Organisations) (Amendment) County — Kyadondo East Regulations. 2020. District — Wakiso PROF. EPHRAIM KAMUNTU (MP), General Notice No. 1216 of 2020. Minister of Justice and Constitutional Affairs. IHE MARRIAGE ACT (Cap. 251 Revised Edition, 2000) General Notice No. 1218 of 2020. THE COMMISSIONERS EOR OATHS NOTICE. (ADVOCATES) ACT COMMISSION place: eor celebration oe marriage (Under Section 5 oftheAct| NOTICE Registry No: 12010000025016-00004 TO ALL TO WHOM THESE PRESEN TS MAY COME, GREETINGS Is i.xi Ruisi of the powers conferred upon me by Section Bi- it known 'i hat on the 27th day 5 of the Marriage Act.