Navigating Birefringence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Second Harmonic Generation in Nonlinear Optical Crystal
Second Harmonic Generation in Nonlinear Optical Crystal Diana Jeong 1. Introduction In traditional electromagnetism textbooks, polarization in the dielectric material is linearly proportional to the applied electric field. However since in 1960, when the coherent high intensity light source became available, people realized that the linearity is only an approximation. Instead, the polarization can be expanded in terms of applied electric field. (Component - wise expansion) (1) (1) (2) (3) Pk = ε 0 (χ ik Ei + χ ijk Ei E j + χ ijkl Ei E j Ek +L) Other quantities like refractive index (n) can be expanded in terms of electric field as well. And the non linear terms like second (E^2) or third (E^3) order terms become important. In this project, the optical nonlinearity is present in both the source of the laser-mode-locked laser- and the sample. Second Harmonic Generation (SHG) is a coherent optical process of radiation of dipoles in the material, dependent on the second term of the expansion of polarization. The dipoles are oscillated with the applied electric field of frequency w, and it radiates electric field of 2w as well as 1w. So the near infrared input light comes out as near UV light. In centrosymmetric materials, SHG cannot be demonstrated, because of the inversion symmetries in polarization and electric field. The only odd terms survive, thus the second order harmonics are not present. SHG can be useful in imaging biological materials. For example, the collagen fibers and peripheral nerves are good SHG generating materials. Since the SHG is a coherent process it, the molecules, or the dipoles are not excited in terms of the energy levels. -

Lecture 14: Polarization
Matthew Schwartz Lecture 14: Polarization 1 Polarization vectors In the last lecture, we showed that Maxwell’s equations admit plane wave solutions ~ · − ~ · − E~ = E~ ei k x~ ωt , B~ = B~ ei k x~ ωt (1) 0 0 ~ ~ Here, E0 and B0 are called the polarization vectors for the electric and magnetic fields. These are complex 3 dimensional vectors. The wavevector ~k and angular frequency ω are real and in the vacuum are related by ω = c ~k . This relation implies that electromagnetic waves are disper- sionless with velocity c: the speed of light. In materials, like a prism, light can have dispersion. We will come to this later. In addition, we found that for plane waves 1 B~ = ~k × E~ (2) 0 ω 0 This equation implies that the magnetic field in a plane wave is completely determined by the electric field. In particular, it implies that their magnitudes are related by ~ ~ E0 = c B0 (3) and that ~ ~ ~ ~ ~ ~ k · E0 =0, k · B0 =0, E0 · B0 =0 (4) In other words, the polarization vector of the electric field, the polarization vector of the mag- netic field, and the direction ~k that the plane wave is propagating are all orthogonal. To see how much freedom there is left in the plane wave, it’s helpful to choose coordinates. We can always define the zˆ direction as where ~k points. When we put a hat on a vector, it means the unit vector pointing in that direction, that is zˆ=(0, 0, 1). Thus the electric field has the form iω z −t E~ E~ e c = 0 (5) ~ ~ which moves in the z direction at the speed of light. -

Lecture 26 – Propagation of Light Spring 2013 Semester Matthew Jones Midterm Exam
Physics 42200 Waves & Oscillations Lecture 26 – Propagation of Light Spring 2013 Semester Matthew Jones Midterm Exam Almost all grades have been uploaded to http://chip.physics.purdue.edu/public/422/spring2013/ These grades have not been adjusted Exam questions and solutions are available on the Physics 42200 web page . Outline for the rest of the course • Polarization • Geometric Optics • Interference • Diffraction • Review Polarization by Partial Reflection • Continuity conditions for Maxwell’s Equations at the boundary between two materials • Different conditions for the components of or parallel or perpendicular to the surface. Polarization by Partial Reflection • Continuity of electric and magnetic fields were different depending on their orientation: – Perpendicular to surface = = – Parallel to surface = = perpendicular to − cos + cos − cos = cos + cos cos = • Solve for /: − = !" + !" • Solve for /: !" = !" + !" perpendicular to cos − cos cos = cos + cos cos = • Solve for /: − = !" + !" • Solve for /: !" = !" + !" Fresnel’s Equations • In most dielectric media, = and therefore # sin = = = = # sin • After some trigonometry… sin − tan − = − = sin + tan + ) , /, /01 2 ) 45/ 2 /01 2 * = - . + * = + * )+ /01 2+32* )+ /01 2+32* 45/ 2+62* For perpendicular and parallel to plane of incidence. Application of Fresnel’s Equations • Unpolarized light in air ( # = 1) is incident -

Lecture 11: Introduction to Nonlinear Optics I
Lecture 11: Introduction to nonlinear optics I. Petr Kužel Formulation of the nonlinear optics: nonlinear polarization Classification of the nonlinear phenomena • Propagation of weak optic signals in strong quasi-static fields (description using renormalized linear parameters) ! Linear electro-optic (Pockels) effect ! Quadratic electro-optic (Kerr) effect ! Linear magneto-optic (Faraday) effect ! Quadratic magneto-optic (Cotton-Mouton) effect • Propagation of strong optic signals (proper nonlinear effects) — next lecture Nonlinear optics Experimental effects like • Wavelength transformation • Induced birefringence in strong fields • Dependence of the refractive index on the field intensity etc. lead to the concept of the nonlinear optics The principle of superposition is no more valid The spectral components of the electromagnetic field interact with each other through the nonlinear interaction with the matter Nonlinear polarization Taylor expansion of the polarization in strong fields: = ε χ + χ(2) + χ(3) + Pi 0 ij E j ijk E j Ek ijkl E j Ek El ! ()= ε χ~ (− ′ ) (′ ) ′ + Pi t 0 ∫ ij t t E j t dt + χ(2) ()()()− ′ − ′′ ′ ′′ ′ ′′ + ∫∫ ijk t t ,t t E j t Ek t dt dt + χ(3) ()()()()− ′ − ′′ − ′′′ ′ ′′ ′′′ ′ ′′ + ∫∫∫ ijkl t t ,t t ,t t E j t Ek t El t dt dt + ! ()ω = ε χ ()ω ()ω + ω χ(2) (ω ω ω ) (ω ) (ω )+ Pi 0 ij E j ∫ d 1 ijk ; 1, 2 E j 1 Ek 2 %"$"""ω"=ω +"#ω """" 1 2 + ω ω χ(3) ()()()()ω ω ω ω ω ω ω + ∫∫d 1d 2 ijkl ; 1, 2 , 3 E j 1 Ek 2 El 3 ! %"$""""ω"="ω +ω"#+ω"""""" 1 2 3 Linear electro-optic effect (Pockels effect) Strong low-frequency -

Understanding Polarization
Semrock Technical Note Series: Understanding Polarization The Standard in Optical Filters for Biotech & Analytical Instrumentation Understanding Polarization 1. Introduction Polarization is a fundamental property of light. While many optical applications are based on systems that are “blind” to polarization, a very large number are not. Some applications rely directly on polarization as a key measurement variable, such as those based on how much an object depolarizes or rotates a polarized probe beam. For other applications, variations due to polarization are a source of noise, and thus throughout the system light must maintain a fixed state of polarization – or remain completely depolarized – to eliminate these variations. And for applications based on interference of non-parallel light beams, polarization greatly impacts contrast. As a result, for a large number of applications control of polarization is just as critical as control of ray propagation, diffraction, or the spectrum of the light. Yet despite its importance, polarization is often considered a more esoteric property of light that is not so well understood. In this article our aim is to answer some basic questions about the polarization of light, including: what polarization is and how it is described, how it is controlled by optical components, and when it matters in optical systems. 2. A description of the polarization of light To understand the polarization of light, we must first recognize that light can be described as a classical wave. The most basic parameters that describe any wave are the amplitude and the wavelength. For example, the amplitude of a wave represents the longitudinal displacement of air molecules for a sound wave traveling through the air, or the transverse displacement of a string or water molecules for a wave on a guitar string or on the surface of a pond, respectively. -

Quartz: a Bull's Eye on Optical Activity
Quartz: a Bull’s Eye on Optical Activity Elise A. Skalwold The Mineralogical Society of America William A. Bassett Title: Quartz: a Bull’s Eye on Optical Activity Authors: Elise Ann Skalwold & William Akers Bassett Edition: First edition Publisher: Mineralogical Society of America, Chantilly, Virginia, USA Copyright: © 2015 by the authors, artists, and photographers. Reproduced with permission. All Rights Reserved. ISBN: 978-0-939950-00-3 Photographer & Designer: Elise A. Skalwold Front cover: Natural quartz crystal 60 x 65 x 40 mm; Hot Springs, Arkansas; ex. Dr. R.W.M. Woodside collection. Back cover: Lab-grown quartz cluster, 140 mm x 90 mm (hydrothermally grown by Mila and Vladimir A. Klipov, R&D XTALS, Inc.). Below: Natural quartz crystals and basal sections. On-going collaboration with Cornell’s Professor Emeritus William A. Bassett is truly priceless to me for this and other projects in the wings, as well as for those over the past eight years of work and research together. Bill shares my enthusi- asm for exploring the fascinating aspects of the classical science of mineralogy, and as my co-author he sets the highest bar for accuracy. All students should be so lucky to have such a mentor. Elise A. Skalwold, 2015 Ithaca, New York Mineralogical Society of America Quartz: a Bull’s Eye on Optical Activity Elise A. Skalwold [email protected] William A. Bassett [email protected] Both Authors: Department of Earth & Atmospheric Sciences Snee Hall, Cornell University Ithaca, NY 14853 All photographs: Elise A. Skalwold Figure 1. The “bull’s eye” uniaxial optic figure characteristic of quartz is indicative of its optical activity. -

Optical Mineralogy in a Nutshell
Optical Mineralogy in a Nutshell Use of the petrographic microscope Slides borrowed/adapted from Jane Selverstone (University of New Mexico) and John Winter (Whitman College) Why use the petrographic microscope? • Identify minerals (no guessing!) • Determine rock type • Determine crystallization sequence • Document deformation history • Observe frozen-in reactions • Constrain P-T history • Note weathering/alteration • Fun, powerful, and cheap! The petrographic microscope Also called a polarizing microscope In order to use the scope, we need to understand a little about the physics of light, and then learn some tools and tricks… Polarized Light Microscopy Isotropic materials, which include gases, liquids, unstressed glasses and cubic crystals, demonstrate the same From Nikon optical properties in all directions. They have only one refractive index and no restriction on the vibration direction of light passing through them. Anisotropic materials, in contrast, which include 90 percent of all solid substances, have optical properties that vary with the orientation of incident light with the crystallographic axes. Anisotropic materials act as beam splitters and divide light rays into two parts. The technique of polarizing microscopy exploits the interference of the split light rays, as they are re-united along the same optical path to extract information about these materials. What happens as light moves through the scope? plane polarised light (single vibration direction) unpolarised light (all possible vibration directions) 1) Light passes -

Syllabus Optical Mineralogy
CRYSTALLOGRAPHY UNIT IV UNIT-IV: SYLLABUS OPTICAL MINERALOGY : Light: Corpuscular, electromagnetic and quantum theories. Ordinary light and plane polarized light. Refractive index an its determination: Relief method, Becke line, Central illumination, and Oblique illumination methods. Isotropism, isotropic minerals and isotropic ray velocity surface. Behaviour of light in isotropic minerals. Petrological Microscope and its parts-optical accessories and their uses:Quartz wedge, Gypsum plate and Mica plate. Study of Isotropic minerals using the petrological microscope: properties of isotropic minerals under parallel Nicol conditions. LightCorpuscular A source of light continuously emits tiny elastic particles called corpuscles. These particles or the corpuscles moves with high velocity as that of light and gets scattered in all directions of light. This theory says that the velocity of light changes with the change in density of the medium in which it is used. This theory could explain three main phenomena of light that is reflection, refraction, and rectilinear propagation of light. This theory also says that the color of light is dependent on the size of the corpuscles. Some of the main drawbacks of this theory are 1) This theory could not explain the phenomena of interference, diffraction, and polarization of light etc. 2) According to this theory the velocity of light in denser medium is greater than the velocity of light in rarer medium but this is proved wrong later 3) This theory assumes that the source of light looses the mass as it emits corpuscles; but not such determent in mass of the source of light is detected. 4) This theory proposes that velocity of the corpuscles increases as the temperature of the source increases as the temperature increases experiments have proved that the velocity of light is independent of temperature. -

GEOL 221 Mineralogy and Mineral Optics Course Syllabus
SAN DIEGO STATE UNIVERSITY GEOL 221 Mineralogy and Mineral Optics Course Syllabus Instructor: Professor David L. Kimbrough Email: [email protected], Phone: 594-1385 Lecture: MW 1000-1050 CSL 422, Lab: W 1400-1640, Lab study: M 1400-1640 CSL 425 Office: GMCS-229A; Office hours: MW 1100-1200; T 1115-1215; by appointment TA: Mark Nahabidian GMCS-133 TBA Course Prerequisite: Chem 200 or concurrent registration. Credit or concurrent registration in OCEAN 100 or GEOL 100 and 101 or GEOL 104 and 101; Geol 200; Required Texts: Introduction to Mineralogy, William D. Nesse, Oxford University Press. Minerals in Thin Section, Perkins & Henke, Prentice Hall Recommended Text: Dictionary of Geological Terms, AGI Bates & Jackson eds. or similar, The Complete Guide to Rocks & Minerals by John Farndon or similar Other required materials: Hand lens; calculator Classroom management: Attendance is crucial. Please let me know if you’re going to be absent for any reason. Be on time for class, don’t participate in excessive side-chatter or cause disruptions during class. Always respond to the instructor, the Teaching Assistant, and your fellow students in a respectful and civil manner. Cheating or plagiarism is not tolerated. It’s easy to spot and constitutes serious academic misconduct. Helpful hints to make Mineralogy easier and more fun! Attend class: Studies show that the most valuable time commitment by students in a course is the time actually spent in the classroom. Class time is the most important determinant of student success and yields the greatest improvement in student learning outcomes. Information is covered in class that is not in the textbook, and parts of the book are hard to understand. -
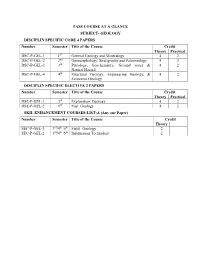
Pass Course at a Glance
PASS COURSE AT A GLANCE SUBJECT- GEOLOGY DISCIPLIN SPECIFIC CORE 4 PAPERS Number Semester Title of the Course Credit Theory Practical DSC-P-GEL-1 1ST General Geology and Mineralogy 4 2 DSC-P-GEL-2 2nd Geomorphology, Stratigraphy and Paleontology 4 2 DSC-P-GEL-3 3rd Petrology, Geochemistry, Ground water & 4 2 Natural Hazard DSC-P-GEL-4 4th Structural Geology, Engineering Geology, & 4 2 Economic Geology DISCIPLIN SPECIFIC ELECTIVE 2 PAPERS Number Semester Title of the Course Credit Theory Practical DSE-P-GEL-1 5th Exploration Geology 4 2 DSE-P-GEL-2 6th Fuel Geology 4 2 SKIL ENHANCEMENT COURSES-LIST-A (Any one Paper) Number Semester Title of the Course Credit Theory SEC-P-GEL-1 3rd/4th /5th Field Geology 2 SEC-P-GEL-2 3rd/4th /5th Information Technology 2 FIRST SEMESTER GEOLOGY PASS PAPER-I Theory Paper-I (General Geology, Crystallography and Mineralogy) Objectives of the Course: The aim of this course is to study General geology part can give an idea about endogenetic process operating inside the earth. study the crystals through external elements of symmetry, crystal classes and systems, and the relations of symmetry to the internal structure using the chemical and physical properties of the minerals. The course aims also to study the major mineral groups, their occurrences, physical, chemical and crystallographic properties and their possible uses in industry. In these units, the physical, chemical and optical properties of the minerals are described. One should know them to identify the types of rocks. Expected outcome: The said courses will make the students to understand about the interior of earth. -

Iceland Spar Calcite: Humidity and Time Effects on Surface Properties and Their Reversibility
This is an electronic reprint of the original article. This reprint may differ from the original in pagination and typographic detail. Wojas, Natalia A.; Swerin, Agne; Wallqvist, Viveca; Järn, Mikael; Schoelkopf, Joachim; Gane, Patrick A.C.; Claesson, Per M. Iceland spar calcite Published in: Journal of Colloid and Interface Science DOI: 10.1016/j.jcis.2019.01.047 Published: 01/04/2019 Document Version Publisher's PDF, also known as Version of record Published under the following license: CC BY-NC-ND Please cite the original version: Wojas, N. A., Swerin, A., Wallqvist, V., Järn, M., Schoelkopf, J., Gane, P. A. C., & Claesson, P. M. (2019). Iceland spar calcite: Humidity and time effects on surface properties and their reversibility. Journal of Colloid and Interface Science, 541, 42-55. https://doi.org/10.1016/j.jcis.2019.01.047 This material is protected by copyright and other intellectual property rights, and duplication or sale of all or part of any of the repository collections is not permitted, except that material may be duplicated by you for your research use or educational purposes in electronic or print form. You must obtain permission for any other use. Electronic or print copies may not be offered, whether for sale or otherwise to anyone who is not an authorised user. Powered by TCPDF (www.tcpdf.org) Journal of Colloid and Interface Science 541 (2019) 42–55 Contents lists available at ScienceDirect Journal of Colloid and Interface Science journal homepage: www.elsevier.com/locate/jcis Iceland spar calcite: Humidity and time effects on surface properties and their reversibility ⇑ Natalia A. -

Handbook for the Deceased: Re-Evaluating Literature and Folklore
HANDBOOK FOR THE DECEASED: RE-EVALUATING LITERATURE AND FOLKLORE IN ICELANDIC ARCHAEOLOGY by BRENDA PREHAL A dissertation submitted to the Graduate Faculty in Anthropology in partial fulfillment of the requirements for the degree of Doctor of Philosophy, The City University of New York. 2021 © 2020 BRENDA PREHAL All rights reserved. ii Handbook for the Deceased: Re-evaluating Literature and Folklore in Icelandic Archaeology by Brenda Prehal This manuscript has been read and accepted for the Graduate Faculty in Anthropology in satisfaction of the dissertation requirement for the degree of Doctor of Philosophy. Date Thomas McGovern Chair of Examining Committee Date Jeff Maskovsky Executive Officer Supervisory Committee: Timothy Pugh Astrid Ogilvie Adolf Frðriksson THE CITY UNIVERSITY OF NEW YORK iii ABSTRACT Handbook for the Deceased: Re-Evaluating Literature and Folklore in Icelandic Archaeology by Brenda Prehal Advisor: Thomas McGovern The rich medieval Icelandic literary record, comprised of mythology, sagas, poetry, law codes and post-medieval folklore, has provided invaluable source material for previous generations of scholars attempting to reconstruct a pagan Scandinavian Viking Age worldview. In modern Icelandic archaeology, however, the Icelandic literary record, apart from official documents such as censuses, has not been considered a viable source for interpretation since the early 20th century. Although the Icelandic corpus is problematic in several ways, it is a source that should be used in Icelandic archaeological interpretation, if used properly with source criticism. This dissertation aims to advance Icelandic archaeological theory by reintegrating the medieval and post-medieval Icelandic literary corpus back into archaeological interpretation. The literature can help archaeologists working in Iceland to find pagan religious themes that span time and place.