Perspectives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

406 | Tutto Arabi - Veterinary Biorhythm
406 | TUTTO ARABI - www.tuttoarabi.com VETERINARY BIORHYTHM by Domenico Bergero, Cynthia Préfontaine Rhythm! • infradian rhythms, which are long-term cycles, such as the To horses, as for men, the passing of time has an impact on annual migration or reproduction cycles found in certain many conscious or automatic activities. When it comes to animals or the human menstrual cycle. men, in particular athletes, we talk about “biorhythm”, i.e. the • ultradian rhythms, which are short cycles, such as the 90-minute effect that longer or shorter cycles can have on performance. REM cycle, the 4-hour nasal cycle, or the 3-hour cycle of But what about our adventure buddy? growth hormone production. They have periods of less than 24 hours. Chronobiology • tidal rhythms, commonly observed in marine life, which follow Chronobiology, from the Greek “chronos” (time) and the (roughly) 12-hour transition from high to low tide “biologia” (study of life), is a field of science that examines and back. periodic (cyclic) phenomena in living organisms and their adaptation to solar and lunar related rhythms. Hormones and physical performance Chronobiological studies include but are not limited to As everyone knows, physical performance is, among other comparative anatomy, physiology, genetics, molecular things, under the control of hormones that determine higher biology and behavior of organisms within biological rhythms or lesser fitness. These hormones can be produced continually mechanics. or, more often than not, can be mero present at certain The variations of the timing and duration of biological times (peak or acrophase) and then decrease. Let’s see which activity in living organisms occur for many essential hormones are involved and what are the best moments for biological processes. -

Profile of Jay C. Dunlap
PROFILE PROFILE Profile of Jay C. Dunlap Paul Gabrielsen Science Writer On moonless nights, the wakes of oceangoing back to oceanography,” Dunlap says, “but I boats sparkle with the blue bioluminescence just thought clocks were the greatest things of unicellular dinoflagellates. As a graduate I’deverheardabout.” He chose to attend student at Harvard University, Jay C. Dunlap Harvard. pondered the carefully orchestrated biological Dunlap found Hastings’ approach to his rhythms that direct dinoflagellates to produce students’ research to be supportive but hands light only at night. Dunlap, a student of off. “He provided all these resources,” Dunlap oceanography at the time, realized that the says, “but he never told people what to do. He field of biological rhythms was still a wide- would give you great feedback on what you open frontier, with many fundamental ques- were doing, but you needed to find your own tions yet to be answered. “This was a place,” way. And if you were lucky enough to do that, he says, “where I could make a mark.” then you really learned how to do science.” Dunlap, Nathan Smith Professor and Chair In 1977, Dunlap attended a 10-week of Genetics at Dartmouth’s Geisel School of summer course on biological rhythms at Medicine and a member of the National Hopkins Marine Station in Pacific Grove, Academy of Sciences since 2009, has devoted California organized by Colin Pittendrigh, his career to answering those fundamental who, along with Hastings, had made pio- questions. His work has uncovered how cir- neering advances in the field of rhythms. The cadian rhythms work at a genetic level, how course attracted scientists studying rhythms environmental cues, such as light, can set bi- along with their graduate students, who, ological clocks, and how the clock can regu- Dunlap says, composed an entire generation Jay C. -

Crystal Structure of the Avirulence Gene Avrlm4-7 of Leptosphaeria Maculans. Illuminates Its Evolutionary and Functional Charact
Crystal structure of the Avirulence Gene AvrLm4-7 of Leptosphaeria maculans. Illuminates its evolutionary and functional characteristics Isabelle Fudal, Francoise Blaise, K Blondeau, M. Graille, A. Labarde, A. Doisy, Bm Tyler, S.D. Kale, Guillaume Daverdin, Marie-Helene Balesdent, et al. To cite this version: Isabelle Fudal, Francoise Blaise, K Blondeau, M. Graille, A. Labarde, et al.. Crystal structure of the Avirulence Gene AvrLm4-7 of Leptosphaeria maculans. Illuminates its evolutionary and functional characteristics. 26. Fungal Genetics Conference at Asilomar, Mar 2011, Asilomar, United States. pp.234. hal-01000740 HAL Id: hal-01000740 https://hal.archives-ouvertes.fr/hal-01000740 Submitted on 6 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 26th Fungal Genetics Conference at Asilomar March 15-20 2011 Principle Financial Sponsors Genetics Society of America Burroughs Wellcome Fund US National Institutes of Health Novozymes Great Lakes Bioenergy Research Center Konkuk University Bio Molecular Informatics Center Genencor, A Danisco Division -

Abstracts from the Neurospora 2002 Conference
Fungal Genetics Reports Volume 49 Article 13 Abstracts from the Neurospora 2002 conference Neurospora 2002 conference Follow this and additional works at: https://newprairiepress.org/fgr This work is licensed under a Creative Commons Attribution-Share Alike 4.0 License. Recommended Citation Neurospora 2002 conference. (2002) "Abstracts from the Neurospora 2002 conference," Fungal Genetics Reports: Vol. 49, Article 13. https://doi.org/10.4148/1941-4765.1195 This Supplementary Material is brought to you for free and open access by New Prairie Press. It has been accepted for inclusion in Fungal Genetics Reports by an authorized administrator of New Prairie Press. For more information, please contact [email protected]. Abstracts from the Neurospora 2002 conference Abstract Abstracts and Poster abstracts from the Neurospora 2002 conference This supplementary material is available in Fungal Genetics Reports: https://newprairiepress.org/fgr/vol49/iss1/13 : Abstracts from the Neurospora 2002 conference Neurospora 2002 Schedule March 14-17, 2002 Invited Abstracts Asilomar Conference Center Pacific Grove, CA. Poster Abstracts SCIENTIFIC PROGRAM Index Barry Bowman Gloria Turner Schedule of Activities Thursday, March 14 3:00 - 6:00 pm, Registration: Administration 6:00 - 7:00 pm, Dinner: Crocker 7:00 - 10:00 pm, Mixer: Kiln Friday, March 15 7:30 - 8:30 am, Breakfast, Crocker 8:30 - 12:00 Noon, Session I, Chapel Genomic Analysis : Mary Anne Nelson, Chair 8:35 - Bruce Birren, MIT, Whitehead Institute. "Genome sequencing for Neurospora crassa." 9:05 - Gertrud Mannhaupt, Heinrich-Heine-University. "The MIPS Neurospora crassa database- MNCDB." 9:30 - Chuck Staben, U. of Kentucky. "Gene finding and annotation for fungal genomes." 9:55 - Alan Radford, University of Leeds. -

Latitudinal Cline of Chronotype
www.nature.com/scientificreports OPEN Latitudinal cline of chronotype Mario André Leocadio-Miguel 1, Fernando Mazzili Louzada2, Leandro Lourenção Duarte3, Roberta Peixoto Areas4, Marilene Alam5, Marcelo Ventura Freire4, John Fontenele-Araujo1, Luiz Menna-Barreto4 & Mario Pedrazzoli4 The rotation of the Earth around its own axis and around the sun determines the characteristics of Received: 15 February 2017 the light/dark cycle, the most stable and ancient 24 h temporal cue for all organisms. Due to the tilt in the earth’s axis in relation to the plane of the earth’s orbit around the sun, sunlight reaches the Earth Accepted: 2 June 2017 differentially depending on the latitude. The timing of circadian rhythms varies among individuals of Published: xx xx xxxx a given population and biological and environmental factors underlie this variability. In the present study, we tested the hypothesis that latitude is associated to the regulation of circadian rhythm in humans. We have studied chronotype profiles across latitudinal cline from around 0° to 32° South in Brazil in a sample of 12,884 volunteers living in the same time zone. The analysis of the results revealed that humans are sensitive to the different sunlight signals tied to differences in latitude, resulting in a morning to evening latitudinal cline of chronotypes towards higher latitudes. The concept of chronotype, which is the expression of diurnal preferences or circadian phenotype, including rise and bedtime preferences, has received a strong consideration from studies in human chronobiology1. It is generally accepted that the chronotype distribution in populations is the same, no matter where the population is geographically localized. -
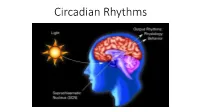
Circadian Rhythms Controlling the Timing of Behaviour by Anticipating the Environment
Circadian Rhythms Controlling the timing of behaviour by anticipating the environment • Circadian = circa + dium • Exists in most if not all unicellular and multicellular organisms The Circadian Circuit Environmental Output Inputs Rhythms Light Hormonal Cycles Temperature Rest/Wake Social Activity Feeding Central Pacemaker Health consequences of circadian misalignment Social Jet Lag Shift Work Increased risk of: - Obesity - Diabetes - Cancer - Mental Illness Light at Night Roenneberg et al. (2012) Current Biology Historical Perspective Jean-Jacques d’Ortous de Mairan (1678 – 1771) Hist de l’Acad Royal Sci (Paris), 1729 “…Il est seulement un peu moins marqué lorsqu’on la tient toujours enfermée dans un lieu obscur…” “The sensitive plant hence perceives the sun without seeing it” Rhythms in leaf-opening persist even in the absence of sunlight Historical Perspective Rat Comp Psychol Monographs, 1922 Nathaniel Kleitman (1895 – 1999) Figure 18.4 Sleep and Wakefulness, 1963 Historical Perspective ’Founders of Chronobiology’ 1960 • Conceptual framework of circadian rhythms • Long before any genes or neural circuits were identified Colin Pittendrigh Jürgen Aschoff (1918 – 1996) (1913 – 1998) Cold Spring Harbor Symposium on Quantitative Biology, Vol. XXV Biological Clocks The Circadian Circuit Environmental Output Inputs Rhythms Light Hormonal Cycles Temperature Rest/Wake Social Activity Feeding Central Pacemaker What would a circadian pacemaker look like? The Circadian Circuit Environmental Output Inputs Rhythms Light Hormonal Cycles Temperature -

Neurospora 2018 OCTOBER 18-21 ASILOMAR CONFERENCE CENTER
PROGRAM and ABSTRACTS Neurospora 2018 OCTOBER 18-21 ASILOMAR CONFERENCE CENTER PACIFIC GROVE CALIFORNIA Cover design by Stephanie Herzog, Technische Universität Braunschweig Neurospora 2018 October 18-21 Asilomar Conference Center Pacific Grove California Scientific Organizers André Fleißner Thomas M. Hammond Technische Universität Braunschweig Illinois State University Neurospora Policy Committee Barry Bowman Jason E. Stajich Molecular Cell & Developmental Biology Dept. Plant Pathology & Microbiology University of California - Santa Cruz University of California - Riverside André Fleißner Thomas M. Hammond Institut für Genetik School of Biological Sciences Technische Universität Braunschweig Illinois State University Brief Schedule Morning Afternoon Evening Thursday Arrival Dinner October 18 Registration Mixer (Heather) Breakfast Lunch Friday Plenary Session I Plenary Session II Dinner October 19 Cell Biology and Metabolism, Signaling and Poster Session Morphogenesis Development Breakfast Lunch Banquet Saturday Plenary Session III Plenary Session IV Speaker October 20 Gene Expression and Genomics, Evolution, and Poster Session Epigenetics Tools Breakfast Sunday Plenary Session V Lunch October 21 Circadian Clocks and Departure Environmental Sensing All Plenary Sessions will be held in Heather. Posters will be displayed in Heather and Toyon throughout the meeting. They should be set up Friday and displayed until the end of the poster session/reception on Saturday evening. Schedule of Activities Thursday, October 18 15:00 - 18:00 p.m. Registration: -

A Fungus Amongst Us 7
Curr. Issues Mol. Biol. 16: 7-8. A Fungus Amongst Us 7 Book review Neurospora: Genomics and Molecular Biology Durgadas P. Kasbekar and Kevin McCluskey (Eds.) Caister Academic Press (2013) ISBN: 978-1-908230-12-6 A Fungus Amongst Us Neurospora Genomics and Molecular Biology Neurospora Genomics and Molecular Biology Neurospora Building on over 70 years of genetics research, Neurospora continues to be the leading model for the study of the genomics and molecular biology of flamentous Genomics and Molecular Biology fungi. The ease of culture, amenability to genetic and molecular genetic analysis, and the close correlation between genetic and biochemical traits are some of its Jennifer Loros advantages. Research with Neurospora has provided insights unachievable from work with simpler systems and difcult to extract from more complicated ones, cementing its position as a leading model system. In recent years the application of modern high throughput analyses had led to a deluge of information on the Department of Biochemistry, Thegenomics andAudrey molecular biology and of Neurospora Theodor. This timely book aims to distil the most important fndings to provide a snapshot of the current research landscape. Geisel School of Medicine at InDartmouth this book, internationally recognizedHanover, Neurospora experts NH, critically review the most important research and demonstrate the breadth of applications to industrial USA biology, biofuels, agriculture, and human health. The opening chapter is an introduction to the organism. Following chapters cover topics such as: carotenoid biosynthesis, polysaccharide deconstruction, genome biology, genome recombination, gene regulation, signal transduction, self-recognition, development, circadian rhythms and mutation. The book closes with a fascinating look at the Durgadas Kasbekar and Kevinhistory and futureMcCluskey trends for research have edited on Neurospora gene and genome Kasbekar and McCluskey Kasbekar an engaging new book calledanalysis. -

Pittendrigh (1918-1996): in Fond Memory
ARTICLE-IH-A-BOX Pittendrigh (1918-1996): In Fond Memory The scientific study of biological rhythms (chronobiology) has really had a many faceted and colourful past in Europe and drew the attention of brilliant naturalists in the 18th, 19th and 20th centuries and is turning out to be one of the most interdiscipli nary branches of modem biology in the 21st century. The conceptual foundations of chronobiology in the 20th century were laid by Jurgen Aschoff, Erwin Blinning and Colin Pittendrigh. Their deep insight and contributions in the second half of that century account for the high degree of scientific elegance and experimental rigour of the subject. In fact, it was the symposium on Biological Clocks convened by Pittendrigh in the Cold Spring Harbor in New York in 1960, with Bunning as the chairperson and the seminal papers of Aschoff and Pittendrigh that ushered in an era of intensified experimental activity. Bunning's address was a definitive version of the history of chronobiology. By 1997, researchers began talking ofa 'party time' for chronobiology since it marked the twenty-fifth anniversary of the discovery of the supra chiasmatic nucleus (SeN) in the brain of mammals, which is the pacemaker of circadian organization, and the identification and cloning of the first circadian clock gene in a mammal. The journal Science, in a December 1998 issue, ranked some of the findings in the field of biological clocks as the first runner-up breakthrough-of-the-year. By 2003 one of our fraternity could write: "The successes achieved by chronobiologists over the past decade have been the envy of the scientific community. -

The Light Mutant Oscillator (Lmo): a Novel Circadian
THE LIGHT MUTANT OSCILLATOR (LMO): A NOVEL CIRCADIAN OSCILLATOR IN NEUROSPORA CRASSA A Thesis by HE HUANG Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE August 2008 Major Subject: Biology THE LIGHT MUTANT OSCILLATOR (LMO): A NOVEL CIRCADIAN OSCILLATOR IN NEUROSPORA CRASSA A Thesis by HE HUANG Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Approved by: Chair of Committee, Deborah Bell-Pedersen Committee Members, Daniel Ebbole Susan Golden Wayne Versaw Head of Department, Vincent Cassone August 2008 Major Subject: Biology iii ABSTRACT The Light Mutant Oscillator (LMO): A Novel Circadian Oscillator in Neurospora crassa. (August 2008) He Huang, B.Eng., Beijing University of Chemical Technology Chair of Advisory Committee: Dr. Deborah Bell-Pedersen Circadian clocks are present in most eukaryotes and some prokaryotes and control rhythms in behavior, physiology and gene expression. One well-characterized circadian clock is that of Neurospora crassa. In addition to the well-described N. crassa FRQ/WCC oscillator, several lines of evidence have implied the presence of other oscillators which may have important functions in the N. crassa circadian clock system. However, the molecular details are only known for the core FRQ/WCC oscillator. The light mutant oscillator (LMO) was identified by two mutations (LM-1 and LM-2) and shown to control developmental rhythms in constant light (LL), conditions in which the FRQ/WCC oscillator is not functional. The objective of this project was to determine whether the developmental rhythms driven by the LMO are circadian, whether the components of the LMO communicate with components of the FRQ/WCC oscillator, and to begin to define the molecular nature of the LMO. -

Neurospora 2004 Conference
Fungal Genetics Reports Volume 51 Article 16 Abstracts from the Neurospora 2004 conference Neurospora Conference Follow this and additional works at: https://newprairiepress.org/fgr This work is licensed under a Creative Commons Attribution-Share Alike 4.0 License. Recommended Citation Neurospora Conference. (2004) "Abstracts from the Neurospora 2004 conference," Fungal Genetics Reports: Vol. 51, Article 16. https://doi.org/10.4148/1941-4765.1146 This Supplementary Material is brought to you for free and open access by New Prairie Press. It has been accepted for inclusion in Fungal Genetics Reports by an authorized administrator of New Prairie Press. For more information, please contact [email protected]. Abstracts from the Neurospora 2004 conference Abstract Abstracts from the Neurospora 2004 conference This supplementary material is available in Fungal Genetics Reports: https://newprairiepress.org/fgr/vol51/iss1/16 : Abstracts from the Neurospora 2004 conference Fungal Genetics Reports Volume 51 Article 16 Abstracts from the Neurospora 2004 conference Neurospora Conference Follow this and additional works at: http://newprairiepress.org/fgr Recommended Citation Neurospora Conference. (2004) "Abstracts from the Neurospora 2004 conference," Fungal Genetics Reports: Vol. 51, Article 16. https://dx.doi.org/10.4148/1941-4765.1146 This Supplementary Material is brought to you for free and open access by New Prairie Press. It has been accepted for inclusion in Fungal Genetics Reports by an authorized administrator of New Prairie Press. For more information, please contact [email protected]. Published by New Prairie Press, 2017 1 Fungal Genetics Reports, Vol. 51 [2004], Art. 16 Abstracts from the Neurospora 2004 conference Abstract Abstracts from the Neurospora 2004 conference Creative Commons License This work is licensed under a Creative Commons Attribution-Share Alike 4.0 License. -

SRBR 2004 Program Book
Ninth Meeting Society for Research on Biological Rhythms Program and Abstracts SRS/SRBR June 23, 2004 SRBR June 24–26, 2004 Whistler Resort • Whistler, British Columbia SOCIETY FOR RESEARCH ON BIOLOGICAL RHYTHMS i Executive Committee Editorial Board Ralph E. Mistleberger Simon Fraser University Steven Reppert, President Serge Daan University of Massachusetts Medical University of Groningen School Larry Morin SUNY, Stony Brook Bruce Goldman William Schwartz, President-Elect University of Connecticut University of Massachusetts Medical Hitoshi Okamura Kobe University School of Medicine School Terry Page Vanderbilt University Carla Green, Secretary Steven Reppert University of Massachusetts Medical University of Virginia Ueli Schibler School University of Geneva Fred Davis, Treasurer Mark Rollag Northeastern University Michael Terman Uniformed Services University Columbia University Helena Illnerova, Member-at-Large Benjamin Rusak Czech. Academy of Sciences Advisory Board Dalhousie University Takao Kondo, Member-at-Large Timothy J. Bartness Nagoya University Georgia State University Laura Smale Michigan State University Anna Wirz-Justice, Member-at-Large Vincent M. Cassone Centre for Chronobiology Texas A & M University Rae Silver Columbia University Journal of Biological Russell Foster Rhythms Imperial College of Science Martin Straume University of Virginia Jadwiga M. Giebultowicz Editor-in-Chief Oregon State University Elaine Tobin Martin Zatz University of California, Los Angeles National Institute of Mental Health Carla Green University of Virginia Fred Turek Associate Editors Northwestern University Eberhard Gwinner Josephine Arendt Max Planck Institute G.T.J. van der Horst University of Surrey Erasmus University Paul Hardin Michael Hastings University of Houston David R. Weaver MRC, Cambridge University of Massachusetts Medical Helena Illerova Center Ken-Ichi Honma Czech.