Pentafluoroethylation of Arenediazonium Tetrafluoroborates Using On‐Site Generated Tetrafluoroethylene
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety Assessment of Polyfluorinated Polymers As Used in Cosmetics
Safety Assessment of Polyfluorinated Polymers as Used in Cosmetics Status: Tentative Report for Public Comment Release Date: June 13, 2018 Panel Date: September 24-25, 2018 All interested persons are provided 60 days from the above date to comment on this safety assessment and to identify additional published data that should be included or provide unpublished data which can be made public and included. Information may be submitted without identifying the source or the trade name of the cosmetic product containing the ingredient. All unpublished data submitted to CIR will be discussed in open meetings, will be available at the CIR office for review by any interested party and may be cited in a peer-reviewed scientific journal. Please submit data, comments, or requests to the CIR Executive Director, Dr. Bart Heldreth. The 2018 Cosmetic Ingredient Review Expert Panel members are: Chair, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Ronald A. Hill, Ph.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; James G. Marks, Jr., M.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Executive Director is Bart Heldreth, Ph.D. This report was prepared by Wilbur Johnson, Jr., M.S., Senior Scientific Analyst and Jinqiu Zhu, Ph.D., Toxicologist. © Cosmetic Ingredient Review 1620 L STREET, NW, SUITE 1200 ◊ WASHINGTON, DC 20036-4702 ◊ PH 202.331.0651 ◊ FAX 202.331.0088 ◊ [email protected] ABSTRACT: The Cosmetic Ingredient Review (CIR) Expert Panel (Panel) reviewed the safety of polyfluorinated polymers in cosmetic products, and most of these ingredients have the film former function in common. -

Miller-Stephenson Material Safety Data Sheet
miller-stephenson Material Safety Data Sheet 1. CHEMICAL PRODUCT/COMPANY IDENTIFICATION Name: MS-122XD Product Use: Release Agent or Dry Lubricant DPMS-Z0612A PTFE Release Agent/Dry Lubricant MANUFACTURER/DISTRIBUTOR: Emergency Phone Number: (800) 424-9300 Milier-Stephenson Chemical George Washington Highway Danbury, Conn. 06810 USA (203) 743-4447 Date Revised: May 2011 2. INGREDIENTS Material (s) CAS No. Approximate % 1,1,1,2-Tetrafluoroethane 811-97-2 80-90 2,3 Dihydroperfluoropentane (HFC-43-lOmee) 138495-42-8 9- 15 Poly-TFE, Omega-Hydro-Alpha-(Methylcyclohexyl)- 65530-85-0 1 -2 Poly-Tetrafluoroethylene 9002-84-0 <1 3. HAZARDS IDENTIFICATION Milky, white, liquid with a faint ethereal odor, packaged in an aerosol container. Potential Health Effects 1,1,1,2-Tetrafluoroethane INHALATION: Misuse or intentional inhalation abuse may cause death without warning symptoms, due to cardiac effects. Other symptoms may include, anaethetic effects, light-headedness, dizziness, confusion, incoordination, drowsiness, or unconsciousness, irregular heartbeat with a strange sensation in the chest, heart thumping, apprehension, feeling of fainting and weakness. Vapors are heavier than air and can cause suffocation by reducing oxygen available for breathing. SKIN: Contact with hquid or refrigerated gas can cause cold bums and frostbite. May cause skin irritation with discomfort, itching, redness or swelling. EYE: Contact with liquid or refrigerated gas can cause cold bums and frostbite. May cause eye irritation with tearing, redness and discomfort. MS-122XD Page 2 of 7 2,3 Dihydroperfluoropentane (HrC-43-lOmee) INHALATION: Gross overexposure by inhalation may cause suffocation if air is displaced by vapors and central nervous system stimulation with increased activity or sleeplessness, tremors or convulsions. -

AROMATIC NUCLEOPHILIC SUBSTITUTION-PART -2 Electrophilic Substitution
Dr. Tripti Gangwar AROMATIC NUCLEOPHILIC SUBSTITUTION-PART -2 Electrophilic substitution ◦ The aromatic ring acts as a nucleophile, and attacks an added electrophile E+ ◦ An electron-deficient carbocation intermediate is formed (the rate- determining step) which is then deprotonated to restore aromaticity ◦ electron-donating groups on the aromatic ring (such as -OH, -OCH3, and alkyl) make the reaction faster, since they help to stabilize the electron-poor carbocation intermediate ◦ Lewis acids can make electrophiles even more electron-poor (reactive), increasing the reaction rate. For example FeBr3 / Br2 allows bromination to occur at a useful rate on benzene, whereas Br2 by itself is slow). In fact, a substitution reaction does occur! (But, as you may suspect, this isn’t an electrophilic aromatic substitution reaction.) In this substitution reaction the C-Cl bond breaks, and a C-O bond forms on the same carbon. The species that attacks the ring is a nucleophile, not an electrophile The aromatic ring is electron-poor (electrophilic), not electron rich (nucleophilic) The “leaving group” is chlorine, not H+ The position where the nucleophile attacks is determined by where the leaving group is, not by electronic and steric factors (i.e. no mix of ortho– and para- products as with electrophilic aromatic substitution). In short, the roles of the aromatic ring and attacking species are reversed! The attacking species (CH3O–) is the nucleophile, and the ring is the electrophile. Since the nucleophile is the attacking species, this type of reaction has come to be known as nucleophilic aromatic substitution. n nucleophilic aromatic substitution (NAS), all the trends you learned in electrophilic aromatic substitution operate, but in reverse. -

The Automated Flow Synthesis of Fluorine Containing Organic Compounds
The automated flow synthesis of fluorine containing organic compounds by CHANTAL SCHOLTZ Submitted in partial fulfilment of the requirements for the degree PHILOSOPHAE DOCTOR In the Faculty of Natural & Agricultural Sciences UNIVERSITY OF PRETORIA PRETORIA Supervisor: Dr D.L. Riley February 2019 DECLARATION I, Chantal Scholtz declare that the thesis/dissertation, which I hereby submit for the degree PhD Chemistry at the University of Pretoria, is my own work and has not previously been submitted by me for a degree at this or any other tertiary institution. Signature :.......................................... Date :..................................... ii ACKNOWLEDGEMENTS I would herewith sincerely like to show my gratitude to the following individuals for their help, guidance and assistance throughout the duration of this project: My supervisor, Doctor Darren Riley, for his knowledge and commitment. Thank you for being a fantastic supervisor and allowing me the opportunity to learn so many valuable skills. My husband, Clinton, for all your love, support and patience and for always being there for me. You are the best. My family, for all the encouragement and support you gave me as well as always believing in me. I will always appreciate what you have done for me. Mr Drikus van der Westhuizen and Mr Johan Postma for their assistance at the Pelchem laboratories with product isolation and characterisation. Dr Mamoalosi Selepe for NMR spectroscopy services, Jeanette Strydom for XRF services and Gerda Ehlers at the UP library for her invaluable assistance. All my friends and colleagues for the continuous moral support, numerous helpful discussions and necessary coffee breaks. My colleagues at Chemical Process Technologies for their ongoing support and motivation, especially Dr Hannes Malan and Prof. -

S.T.E.T.Women's College, Mannargudi Semester Iii Ii M
S.T.E.T.WOMEN’S COLLEGE, MANNARGUDI SEMESTER III II M.Sc., CHEMISTRY ORGANIC CHEMISTRY - II – P16CH31 UNIT I Aliphatic nucleophilic substitution – mechanisms – SN1, SN2, SNi – ion-pair in SN1 mechanisms – neighbouring group participation, non-classical carbocations – substitutions at allylic and vinylic carbons. Reactivity – effect of structure, nucleophile, leaving group and stereochemical factors – correlation of structure with reactivity – solvent effects – rearrangements involving carbocations – Wagner-Meerwein and dienone-phenol rearrangements. Aromatic nucleophilic substitutions – SN1, SNAr, Benzyne mechanism – reactivity orientation – Ullmann, Sandmeyer and Chichibabin reaction – rearrangements involving nucleophilic substitution – Stevens – Sommelet Hauser and von-Richter rearrangements. NUCLEOPHILIC SUBSTITUTION Mechanism of Aliphatic Nucleophilic Substitution. Aliphatic nucleophilic substitution clearly involves the donation of a lone pair from the nucleophile to the tetrahedral, electrophilic carbon bonded to a halogen. For that reason, it attracts to nucleophile In organic chemistry and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which a leaving group(nucleophile) is replaced by an electron rich compound(nucleophile). The whole molecular entity of which the electrophile and the leaving group are part is usually called the substrate. The nucleophile essentially attempts to replace the leaving group as the primary substituent in the reaction itself, as a part of another molecule. The most general form of the reaction may be given as the following: Nuc: + R-LG → R-Nuc + LG: The electron pair (:) from the nucleophile(Nuc) attacks the substrate (R-LG) forming a new 1 bond, while the leaving group (LG) departs with an electron pair. The principal product in this case is R-Nuc. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. -

Perfluorooctanoic Acid (PFOA)
PERFLUOROOCTANOIC ACID 1. Exposure Data 1.1.2 Structural and molecular formulae, and relative molecular mass: straight-chain 1.1 Identification of the agent isomer 1.1.1 Nomenclature F FFF F F F O F Chem. Abstr. Serv. Reg. No.: 335-67-1 OH Chem. Abstr. Serv. Name: Perfluorooctanoic FFF F F F F acid Molecular formula: C8HF15O2 IUPAC Name: 2,2,3,3,4,4,5,5,6,6,7,7,8,8,8- Relative molecular mass: 414 pentadecafluorooctanoic acid Synonyms: PFOA; pentadecafluoro-1-octan - 1.1.3 Chemical and physical properties of the oic acid; pentadecafluoro-n-octanoic acid; pure substance: straight-chain isomer pentadecaflurooctanoic acid; perfluoro- caprylic acid; perfluoroctanoic acid; From HSDB (2014), unless otherwise perfluoroheptanecarboxylic acid; APFO; indicated ammonium perfluorooctanoate Description: White to off-white powder Isomers and Salts: There are 39 possible struc- Boiling point: 192.4 °C tural isomers of pentadecafluorooctanoic acid (1 with chain length 8, 5 with chain Melting point: 54.3 °C length 7, 13 with chain length 6, 16 with chain Density: 1.792 g/cm3 at 20 °C length 5, and 4 with chain length 4). These Solubility: 9.5 g/L in water at 25 °C isomers can also exist as the ammonium, Vapour pressure: 0.0023 kPa at 20 °C (extrap- sodium, or potassium salt (Nielsen, 2012). olated); 0.127 kPa at 59.25 °C (measured) Fig. 1.1 presents the few isomers and salts (ATSDR, 2009); 0.070 kPa at 25 °C that have Chemical Abstracts Service (CAS) Stability: When heated to decomposition it references. -

DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS
criteria for a recommended standard . occupational exposure to DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE criteria for a recommended standard... OCCUPATIONAL EXPOSURE TO DECOMPOSITION PRODUCTS of FLUOROCARBON POLYMERS U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service Center for Disease Control National Institute for Occupational Safety and Health September 1977 For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 DHEW (NIOSH) Publication No. 77-193 PREFACE The Occupational Safety and Health Act of 1970 emphasizes the need for standards to protect the health and safety of workers exposed to an ever-increasing number of potential hazards at their workplace. The National Institute for Occupational Safety and Health has projected a formal system of research, with priorities determined on the basis of specified indices, to provide relevant data from which valid criteria for effective standards can be derived. Recommended standards for occupational exposure, which are the result of this work, are based on the health effects of exposure. The Secretary of Labor will weigh these recommendations along with other considerations such as feasibility and means of implementation in developing regulatory standards. It is intended to present successive reports as research and epidemiologic studies are completed and as sampling and analytical methods are developed. Criteria and standards will be reviewed periodically to ensure continuing protection of the worker. I am pleased to acknowledge the contributions to this report on the decomposition products of fluorocarbon polymers by members of the NIOSH staff and the valuable constructive comments by the Review Consultants on the Decomposition Products of Fluorocarbon Polymers and by Robert B. -

Synthesis of Organobromines As a Tool for Their Characterisation and Environmental Occurrence Assessment
Synthesis of organobromines as a tool for their characterisation and environmental occurrence assessment Andreas Rydén Department of Materials and Environmental Chemistry Stockholm University Stockholm 2013 i Doctoral Thesis 2013 Department of Materials and Environmental Chemistry Stockholm University SE-106 91 Stockholm Sweden Abstract Polybrominated diphenyl ethers (PBDEs) have been intensively used as flame retardants (FRs) and have become ubiquitous environmental pollutants. PBDEs form hydroxylated PBDEs (OH-PBDEs) as metabolites. Further, some OH-PBDEs and methoxy-PBDEs (MeO-PBDEs) are natural products. These are all compounds of environmental and health concern and it is therefore important to confirm their identity and to assess their environmental levels and toxicities. Hence, it is vital to obtain authentic reference standards of individual PBDEs and OH/MeO-PBDEs. The thesis main aim was to develop synthesis methods of congener specific PBDEs, OH- and MeO-PBDEs. The second aim was to identify and quantify PBDEs, OH- and MeO-PBDEs in environmental samples. The third was to propose an abbreviation system for FRs. O-Arylation of brominated phenols, using either symmetrical or unsymmetrical brominated diphenyliodonium salts, was selected for synthesis of PBDEs and OH- /MeO-PBDEs. A total of 16 MeO-PBDEs, 11 OH-PBDEs, 1 diMeO-PBDE and 1 EtO-MeO-PBDE were synthesised. Three novel unsymmetrical diaryliodonium triflates were synthesised and used in synthesis. Optimisations were made to construct a reliable general method for congener specific PBDE synthesis, which was used in the synthesis of 8 representative PBDE congeners. The products were generally characterised by electron ionisation mass spectrometry (EIMS) and nuclear magnetic resonance (NMR) spectroscopy. -
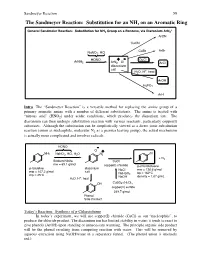
The Sandmeyer Reaction: Substitution for an NH2 on an Aromatic Ring
Sandmeyer Reaction 59 The Sandmeyer Reaction: Substitution for an NH2 on an Aromatic Ring + General Sandmeier Reaction: Substitution for NH2 Group on a Benzene, via Diazonium ArN2 ArCN CuCN CuBr ArBr NaNO2, HCl HONO + - CuCl ArNH2 ArN2 Cl ArCl diazonium salt + H2O, H , heat ArOH H3PO2 ArH Intro The “Sandmeyer Reaction” is a versatile method for replacing the amine group of a primary aromatic amine with a number of different substitutents. The amine is treated with “nitrous acid” (HNO2) under acidic conditions, which produces the diazonium ion. The diazonium can then undergo substitution reaction with various reactants, particularly copper(I) substrates. Although the substitution can be simplistically viewed as a direct ionic substitution reaction (anion as nucleophile, molecular N2 as a premier leaving group), the actual mechanism is actually more complicated and involves radicals. HONO - Cl + NH2 NaNO2, HCl, H2O N2 Cl + N2 Sodium Nitrite CuCl mw = 69.1 g/mol copper(I) chloride p-chlorotoluene p-toluidine diazonium NaCl mw = 126.6 g/mol mw = 107.2 g/mol salt NaHSO bp = 162ºC mp = 45ºC 3 + NaOH density = 1.07 g/mL H2O, H , heat OH CuSO4-(H2O)5 copper(II) sulfate 249.7 g/mol Phenol Side Product Today’s Reaction: Synthesis of p-Chlorotoluene In today’s experiment, we will use copper(I) chloride (CuCl) as our “nucleophile”, to produce the chloride product. The diazonium ion has limited stability in water; it tends to react to give phenols (ArOH) upon standing or unnecessary warming. The principle organic side product will be the phenol resulting from competing reaction with water. -

Tetrafluoroethylene
TETRAFLUOROETHYLENE Tetrafluoroethylene was reviewed previously by the Working Group in 1979, 1987, and 1998 (IARC, 1979, 1987, 1999). New data have since become available, and these have been incor- porated, and taken into consideration in the present evaluation. 1. Exposure Data 1.1.3 Chemical and physical properties of the pure substance 1.1 Identification of the agent From IFA (2014), unless otherwise indicated 1.1.1 Nomenclature Description: Colourless gas, odourless or sometimes described as having a faint Chem. Abstr. Serv. Reg. No.: 116-14-3 sweetish odour; extremely flammable Chem. Abstr. Serv. Name: Tetrafluoroethylene Boiling point: −75.63 °C IUPAC Systematic Name: Melting point: −131.15 °C (HSDB, 2014) 1,1,2,2-Tetrafluoroethene Density: 4216 kg/m3 at 15 °C at 1 bar Synonyms: Perfluoroethylene, Perfluoroethene, Solubility: Slightly soluble in water, 159 mg/L Ethylene tetrafluoro-, tetrafluoroethene at 25 °C (HSDB, 2014) Vapour pressure: 2947 kPa and 20 °C 1.1.2 Structural and molecular formulae, and Stability: Decomposes into fluorine and fluo- relative molecular mass rine compounds when heated (HSDB, 2014) Reactivity: A terpene inhibitor (limonene) is F F generally added to the monomer to prevent CC spontaneous polymerization. F F Risk of explosion in contact with air or in the absence of air at elevated temperatures and/or Molecular formula: C2F4 pressures (> 600 °C and 100 kPa). The stabilized Relative molecular mass: 100.01 monomer is flammable in air if ignited (flamma- bility limits: lower, 11%; upper, 60%) producing soot and carbon tetrafluoride Babenko( et al., 1993; HSDB, 2014). Incompatible with polymerization catalysts and peroxides. -

Freon™ 123 PUSH Bulletin
Freon™ 123 Refrigerant (R-123) Properties, Uses, Storage, and Handling Freon™ 123 Refrigerant Table of Contents Shipping, Storage, and Handling ......................................16 Shipping Containers in the United States ..............................16 Introduction ...........................................................................3 Bulk Storage Systems ..........................................................................16 Background .......................................................................................................... 3 Storage, Handling, and Use Recommendations .................16 Freon™ 123—An Environmentally Acceptable Prohibited Uses ...................................................................................16 Alternative ....................................................................................................... 3 Personal Protective Equipment ................................................17 Uses .......................................................................................3 Storage ......................................................................................................17 Refrigerant ...................................................................................................... 4 Handling ....................................................................................................17 Heat-Transfer Fluid ....................................................................................4 Charging, Maintenance, and Sampling .................................18 -

Teflon™ FEP Fluoropolymer Resins
Teflon™ FEP Fluoropolymer Resins Product and Properties Handbook Teflon™ FEP Fluoropolymer Resins Table of Contents Introduction . 3 Commercially Available Products . 3 Specifications . 3 General Properties of Teflon™ FEP . 3 Typical Properties of Teflon™ FEP . 3 Mechanical Properties . 3 Tensile Properties . 4 Flexural Modulus . 4 Compressive Stress . 5 Creep and Cold Flow . 5 Apparent Modulus of Elasticity . 5 Stress Relaxation . 5 Poisson’s Ratio . 9 Fatigue Resistance . 9 Impact Resistance . 9 Hardness . 10 Friction . 10 Thermal Properties . 10 Electrical Properties . 11. Chemical Resistance . 11 Absorption . 11 Permeability . 11 Weathering . 11 Cryogenic Service . .11 Mildew Resistance . 13 FDA Compliance . 13 Optical Properties . 13 Fabrication Techniques . 13 Forming and Fabrication . 14 Choose Correct Working Speeds . 14 Properly Shape and Use Tools . 14 Rules for Dimensioning and Finishing . 15 Closer Tolerances . 15 Measuring Tolerances . 15 Surface Finishes . 15 Safety Precautions . 15 2 Teflon™ FEP Fluoropolymer Resins Introduction Specifications Teflon™ FEP (fluorinated ethylene propylene) is The ASTM material specification covering Teflon™ FEP chemically a copolymer of hexafluoropropylene fluoropolymer resin is D2116. Teflon™ FEP fluoropolymer and tetrafluoroethylene. It differs from PTFE resin is also called out in various industrial and military (polytetrafluoroethylene) resins, in that it is melt- specifications for tubing, molded parts, and film, as well as processible using conventional injection molding and numerous