POU Reverse Osmosis Performance and Sizing (Excerpted from WQA’S Online Knowledge Base) Reverse Osmosis Performance Factors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reverse Osmosis Bibliography Additional Readings
8/24/2016 Osmosis AccessScience from McGrawHill Education (http://www.accessscience.com/) Osmosis Article by: Johnston, Francis J. Department of Chemistry, University of Georgia, Athens, Georgia. Publication year: 2016 DOI: http://dx.doi.org/10.1036/10978542.478400 (http://dx.doi.org/10.1036/10978542.478400) Content Osmotic pressure Reverse osmosis Bibliography Additional Readings The transport of solvent through a semipermeable membrane separating two solutions of different solute concentration. The solvent diffuses from the solution that is dilute in solute to the solution that is concentrated. The phenomenon may be observed by immersing in water a tube partially filled with an aqueous sugar solution and closed at the end with parchment. An increase in the level of the liquid in the solution results from a flow of water through the parchment into the solution. The process occurs as a result of a thermodynamic tendency to equalize the sugar concentrations on both sides of the barrier. The parchment permits the passage of water, but hinders that of the sugar, and is said to be semipermeable. Specially treated collodion and cellophane membranes also exhibit this behavior. These membranes are not perfect, and a gradual diffusion of solute molecules into the more dilute solution will occur. Of all artificial membranes, a deposit of cupric ferrocyanide in the pores of a finegrained porcelain most nearly approaches complete semipermeability. The walls of cells in living organisms permit the passage of water and certain solutes, while preventing the passage of other solutes, usually of relatively high molecular weight. These walls act as selectively permeable membranes, and allow osmosis to occur between the interior of the cell and the surrounding media. -

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Solute Concentration: Molality
5/25/2012 Colligative Properties of Solutions . Colligative Properties: • Solution properties that depend on concentration of solute particles, not the identity of particles. Previous example: vapor pressure lowering. Consequences: change in b.p. and f.p. of solution. © 2012 by W. W. Norton & Company Solute Concentration: Molality . Changes in boiling point/freezing point of solutions depends on molality: moles of solute m kg of solvent • Preferred concentration unit for properties involving temperature changes because it is independent of temperature. © 2012 by W. W. Norton & Company 1 5/25/2012 Calculating Molality Starting with: a) Mass of solute and solvent. b) Mass of solute/ volume of solvent. c) Volume of solution. © 2012 by W. W. Norton & Company Sample Exercise 11.8 How many grams of Na2SO4 should be added to 275 mL of water to prepare a 0.750 m solution of Na2SO4? Assume the density of water is 1.000 g/mL. © 2012 by W. W. Norton & Company 2 5/25/2012 Boiling-Point Elevation and Freezing-Point Depression . Boiling Point Elevation (ΔTb): • ΔTb = Kb∙m • Kb = boiling point elevation constant of solvent; m = molality. Freezing Point Depression (ΔTf): • ΔTf = Kf∙m • Kf = freezing-point depression constant; m = molality. © 2012 by W. W. Norton & Company Sample Exercise 11.9 What is the boiling point of seawater if the concentration of ions in seawater is 1.15 m? © 2012 by W. W. Norton & Company 3 5/25/2012 Sample Exercise 11.10 What is the freezing point of radiator fluid prepared by mixing 1.00 L of ethylene glycol (HOCH2CH2OH, density 1.114 g/mL) with 1.00 L of water (density 1.000 g/mL)? The freezing-point-depression constant of water, Kf, is 1.86°C/m. -

The New Formula That Replaces Van't Hoff Osmotic Pressure Equation
New Osmosis Law and Theory: the New Formula that Replaces van’t Hoff Osmotic Pressure Equation Hung-Chung Huang, Rongqing Xie* Department of Neurosciences, University of Texas Southwestern Medical Center, Dallas, TX *Chemistry Department, Zhengzhou Normal College, University of Zhengzhou City in Henan Province Cheng Beiqu excellence Street, Zip code: 450044 E-mail: [email protected] (for English communication) or [email protected] (for Chinese communication) Preamble van't Hoff, a world-renowned scientist, studied and worked on the osmotic pressure and chemical dynamics research to win the first Nobel Prize in Chemistry. For more than a century, his osmotic pressure formula has been written in the physical chemistry textbooks around the world and has been generally considered to be "impeccable" classical theory. But after years of research authors found that van’t Hoff osmotic pressure formula cannot correctly and perfectly explain the osmosis process. Because of this, the authors abstract a new concept for the osmotic force and osmotic law, and theoretically derived an equation of a curve to describe the osmotic pressure formula. The data curve from this formula is consistent with and matches the empirical figure plotted linearly based on large amounts of experimental values. This new formula (equation for a curved relationship) can overcome the drawback and incompleteness of the traditional osmotic pressure formula that can only describe a straight-line relationship. 1 Abstract This article derived a new abstract concept from the osmotic process and concluded it via "osmotic force" with a new law -- "osmotic law". The "osmotic law" describes that, in an osmotic system, osmolyte moves osmotically from the side with higher "osmotic force" to the side with lower "osmotic force". -

Osmosis and Osmoregulation Robert Alpern, M.D
Osmosis and Osmoregulation Robert Alpern, M.D. Southwestern Medical School Water Transport Across Semipermeable Membranes • In a dilute solution, ∆ Τ∆ Jv = Lp ( P - R CS ) • Jv - volume or water flux • Lp - hydraulic conductivity or permeability • ∆P - hydrostatic pressure gradient • R - gas constant • T - absolute temperature (Kelvin) • ∆Cs - solute concentration gradient Osmotic Pressure • If Jv = 0, then ∆ ∆ P = RT Cs van’t Hoff equation ∆Π ∆ = RT Cs Osmotic pressure • ∆Π is not a pressure, but is an expression of a difference in water concentration across a membrane. Osmolality ∆Π Σ ∆ • = RT Cs • Osmolarity - solute particles/liter of water • Osmolality - solute particles/kg of water Σ Osmolality = asCs • Colligative property Pathways for Water Movement • Solubility-diffusion across lipid bilayers • Water pores or channels Concept of Effective Osmoles • Effective osmoles pull water. • Ineffective osmoles are membrane permeant, and do not pull water • Reflection coefficient (σ) - an index of the effectiveness of a solute in generating an osmotic driving force. ∆Π Σ σ ∆ = RT s Cs • Tonicity - the concentration of effective solutes; the ability of a solution to pull water across a biologic membrane. • Example: Ethanol can accumulate in body fluids at sufficiently high concentrations to increase osmolality by 1/3, but it does not cause water movement. Components of Extracellular Fluid Osmolality • The composition of the extracellular fluid is assessed by measuring plasma or serum composition. • Plasma osmolality ~ 290 mOsm/l Na salts 2 x 140 mOsm/l Glucose 5 mOsm/l Urea 5 mOsm/l • Therefore, clinically, physicians frequently refer to the plasma (or serum) Na concentration as an index of extracellular fluid osmolality and tonicity. -
Mol of Solute Molality ( ) = Kg Solvent M
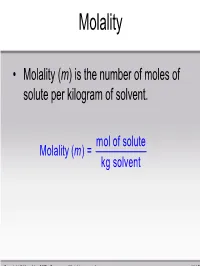
Molality • Molality (m) is the number of moles of solute per kilogram of solvent. mol of solute Molality (m ) = kg solvent Copyright © Houghton Mifflin Company. All rights reserved. 11 | 51 Sample Problem Calculate the molality of a solution of 13.5g of KF dissolved in 250. g of water. mol of solute m = kg solvent 1 m ol KF ()13.5g 58.1 = 0.250 kg = 0.929 m Copyright © Houghton Mifflin Company. All rights reserved. 11 | 52 Mole Fraction • Mole fraction (χ) is the ratio of the number of moles of a substance over the total number of moles of substances in solution. number of moles of i χ = i total number of moles ni = nT Copyright © Houghton Mifflin Company. All rights reserved. 11 | 53 Sample Problem -Conversions between units- • ex) What is the molality of a 0.200 M aluminum nitrate solution (d = 1.012g/mL)? – Work with 1 liter of solution. mass = 1012 g – mass Al(NO3)3 = 0.200 mol × 213.01 g/mol = 42.6 g ; – mass water = 1012 g -43 g = 969 g 0.200mol Molality==0.206 mol / kg 0.969kg Copyright © Houghton Mifflin Company. All rights reserved. 11 | 54 Sample Problem Calculate the mole fraction of 10.0g of NaCl dissolved in 100. g of water. mol of NaCl χ=NaCl mol of NaCl + mol H2 O 1 mol NaCl ()10.0g 58.5g NaCl = 1 m ol NaCl 1 m ol H2 O ()10.0g+ () 100.g H2 O 58.5g NaCl 18.0g H2 O = 0.0299 Copyright © Houghton Mifflin Company. -

The Physics of Osmotic Pressure
bioRxiv preprint doi: https://doi.org/10.1101/116806; this version posted March 14, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. The physics of osmotic pressure M. G. Bowler Department of Physics, University of Oxford, Keble Road, Oxford OX1 3RH a) Abstract The proper application of the virial theorem to the origin of osmotic pressure provides a simple, vivid and complete explanation of the physical processes involved; osmosis is driven by differential solvent pressures. This simple and intuitive notion is widely disregarded, but the virial theorem allows an exact formulation and unifies a number of different treatments. It is closely related to a kinetic treatment devised by Ehrenfest over 100 years ago. I. INTRODUCTION Consider two reservoirs, one filled with a solvent such as pure water and the other with a solution, such as salt or sugar in water (Fig.1). If the two are separated only by a semi-permeable membrane that passes water in both PL PR L R Figure 1 shows two compartments separated by a semi-permeable membrane. The left contains pure solvent (water) and the right contains solution (such as salt in water). Water streams from left to right until the right hand pressure exceeds the left hand pressure by the osmotic pressure. Fij directions but does not pass salt at all, then water streamsFin into the solution and the flow is only arrested if the external pressure applied to the solution exceeds w w that applied to the solvent by the osmotic pressure Π. -

A New Approach to Derivation of Van't Hoff Equation for Osmotic Pressure of a Dilute Solution
American International Journal of Available online at http://www.iasir.net Research in Science, Technology, Engineering & Mathematics ISSN (Print): 2328-3491, ISSN (Online): 2328-3580, ISSN (CD-ROM): 2328-3629 AIJRSTEM is a refereed, indexed, peer-reviewed, multidisciplinary and open access journal published by International Association of Scientific Innovation and Research (IASIR), USA (An Association Unifying the Sciences, Engineering, and Applied Research) A new approach to derivation of Van’t Hoff equation for osmotic pressure of a dilute solution Rita Khare Department of Chemistry, Government Women’s College, Gardanibagh, Patna, Bihar, INDIA. Abstract: Relative lowering of vapour pressure which is a colligative property of dilute solution of non volatile solute in a volatile solvent has been determined theoretically by applying conditions of Raoult’s law. Other colligative properties also have theoretical basis but osmotic pressure which is also a colligative property is expressed by an equation which was deduced empirically by Van’t Hoff. Van’t Hoff’s theory of dilute solutions supports the view that solute particles in a dilute solution behave in a manner similar to that of gas molecules in a gas i.e solute molecules obey Boyle’s law, Charles law and Avogadro’s law. In this paper it is suggested that it is not the solute molecules but volatile solvent molecules present in a dilute solution that behave like gas molecules in a gas. Here an attempt is made to derive Van’t Hoff equation by applying Raoult’s law and by considering the pressure exerted by molecules of the solvent in liquid state on the basis of proposed “theory of solvent pressure”. -

Osmotic Pressure of Confined Square Lattice Self-Avoiding Walks
Osmotic Pressure of Confined Square Lattice Self-Avoiding Walks F Gassoumov1y & EJ Janse van Rensburg1z 1Department of Mathematics and Statistics, York University, Toronto, Ontario M3J 1P3, Canada Abstract. Flory-Huggins theory is a mean field theory for modelling the free energy of dense polymer solutions and polymer melts. In this paper we use Flory-Huggins theory as a model of a dense two dimensional self-avoiding walk compressed in a square in the square lattice. The theory describes the free energy of the walk well, and we estimate the Flory interaction parameter of the walk to be χsaw = 0:32(1). arXiv:1806.01746v3 [cond-mat.stat-mech] 17 Aug 2018 y [email protected] z [email protected] Osmotic Pressure of Confined Square Lattice Self-Avoiding Walks 2 1. Introduction Flory-Huggins theory [7] is used widely in the modelling of polymer solutions and polymer melts. The basic component in this theory is the mean field quantification of the entropy of a polymer. If a single chain is considered, then the entropy per unit volume V is estimated by φ φ −Ssite(φ) = N log N + (1 − φ) log(1 − φ) (1) N where φ = V is the volume fraction (or concentration) of monomers in a chain of length N (degree of polymerization) confined in a space of volume V . In Flory-Huggins theory the full entropy Ssite(φ) is modified to the entropy of mixing Smix [6] per site, which is the difference between Ssite(φ) and the weighted average of the entropy of pure solvent Ssite(0), and pure polymer Ssite(1): φ −Smix = −Ssite(φ) + (1 − φ)Ssite(0) + φ Ssite(1) = N log φ + (1 − φ) log(1 − φ): (2) Notice the cancellation in Smix of the term linear in φ. -

About Reverse Osmosis (Ro)
ABOUT REVERSE OSMOSIS (RO) WHAT IS REVERSE OSMOSIS (RO)? (SUMMARY) Reverse Osmosis (RO) is a membrane separation process in which feed water flows along the membrane surface under pressure. Purified water permeates the membrane and is collected, while the concentrated water, containing dissolved and undissolved material that does not flow through the membrane, is discharged to the drain. The key requirements of Reverse Osmosis (RO) process are a membrane and water under pressure. Other requirements include prefiltration to remove suspended impurities and carbon to remove chlorine (damages the membrane). Most membranes remove 90-99+ % of the dissolved impurities depending on the impurity and the composition of water. Reverse osmosis systems (RO Systems) remove salts, microorganisms and many high molecular weight organics. System capacity depends on the water temperature, total dissolved solids in feed water, operating pressure and the overall recovery of the system. BENEFITS OF REVERSE OSMOSIS (RO) REVERSE OSMOSIS RO SYSTEM REMOVES UP TO 99% OF TOTAL DISSOLVED SOLIDS Reverse osmosis is a membrane separation process in which feed water flows along the membrane surface under pressure. Purified water permeates the membrane and is collected, while the concentrated water, containing dissolved and undissolved material that does not flow through the membrane, is discharged to the drain. Reverse osmosis systems remove salts, microorganisms and many high molecular weight organics. System capacity depends on the water temperature, total dissolved solids in feed water, operating pressure and the overall recovery of the system. ADVANTAGES OF REVERSE OSMOSIS OVER CONVENTIONAL PROCESSES Compared with other conventional water treatment processes, reverse osmosis has proven to be the most efficient means of removing salts, chemical contaminants and heavy metals, such as lead, from drinking water. -

Reverse Osmosis
Reverse Osmosis Presented by Dick Youmans, CWT David H. Paul, Inc. 2 Outline for this Presentation 1) Define Osmosis and Reverse Osmosis 2) Types of Membranes and Their Characteristics 3) Manufacturing Process for the “Membrane” 4) Demonstration: Rolling One Of Our Own 5) Understand Water Flow Through an Element 6) Understand Water Flow Through Pressure Vessels 3 Definition of Osmosis Osmosis is the process where a solvent (usually water) passes from a dilute solution into a more concentrated one by moving through a semipermeable membrane which selectively allows the passage of the solvent, but restricts the passage of the solute (dissolved solids). 4 Osmosis Time = Zero Equilibrium Time Less Concentrated Pure Concentrated Pure Solution Water Solution Water 5 Osmosis 6 Osmosis Time = Zero Equilibrium Time Less Concentrated Pure Concentrated Pure Solution Water Solution Water 7 Types of Pressures Involved 1) Osmotic Pressure 2) Applied Pressures A) Hydrostatic or Head Pressure B) Pump or External Pressure 3) Net Driving Pressure 8 Osmotic Pressure Time = Zero Equilibrium Time <1000 ppm 1000 Pure ppm TDS TDS Pure Water Water Osmotic Pressure 10 psi 9 Approximate Osmotic Pressures • TDS in ppm • Osmotic Pressure in psi (bar) 100 ppm 1 psi (.069Bar) 1,000 ppm 10 psi (.69 Bar) 5,000 ppm 50 psi (3.45 Bar) 10,000 ppm 100 psi 6.9 Bar) 15,000 ppm 150 psi (10.35 Bar) 10 Net Osmotic Pressure Time = Zero Equilibrium Time 1000 1000 1000 1000 ppm ppm ppm ppm TDS TDS TDS TDS Osmotic Pressure 10 psi 10 psi Net Osmotic Pressure is 0 psi 11 Applied Pressure -

1 051407 Quiz 6 Polymer Properties 1) in Class We Discussed The
051407 Quiz 6 Polymer Properties 1) In class we discussed the similarity between an ideal gas and a polymer solution using the ideal gas law, P = ρkT, where ρ = n/V and n is the number of gas atoms in the system. a) Does the ideal gas law apply to an incompressible or to a compressible system? b) The Flory-Huggins equation involves the log of the volume fraction. Show that the thermodynamic expression, dGmixing = Vmixing dP can be used to obtain an expression similar to the Flory-Huggins entropy of mixing term, ΔGmixing = kT ln(Pmixture/Ppure). c) For an ideal gas the partial pressure is equal to the molar volume. Is this identity appropriate for an incompressible system or for a compressible system? d) Explain why equations for gasses (compressible systems) could be appropriate for highly incompressible systems (polymers). e). Engraved on Boltzman’s tomb in Vienna is S = k lnΩ, where Ω is the number of states that a system can achieve. If Ω = n!/(n1! n2!) and using Sterling’s approximation, ln y! = y lny - y for large y, show that you can obtain an expression similar to the Flory- Huggins Entropy of mixing and to the ideal gas mixing law. 2) Explain how a polymer in an osmometer is similar to an ideal gas by: a) Sketch an osmometer and describe the source of the excess pressure on the polymer side. b) Write a virial expansion (Taylor Power Series expansion) in concentration from the ideal gas law. c) Compare this expression with the expression obtained from the Flory-Huggins Equation, π = kT c [1/N + (1/2 - χ) c].