The New Formula That Replaces Van't Hoff Osmotic Pressure Equation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reverse Osmosis Bibliography Additional Readings
8/24/2016 Osmosis AccessScience from McGrawHill Education (http://www.accessscience.com/) Osmosis Article by: Johnston, Francis J. Department of Chemistry, University of Georgia, Athens, Georgia. Publication year: 2016 DOI: http://dx.doi.org/10.1036/10978542.478400 (http://dx.doi.org/10.1036/10978542.478400) Content Osmotic pressure Reverse osmosis Bibliography Additional Readings The transport of solvent through a semipermeable membrane separating two solutions of different solute concentration. The solvent diffuses from the solution that is dilute in solute to the solution that is concentrated. The phenomenon may be observed by immersing in water a tube partially filled with an aqueous sugar solution and closed at the end with parchment. An increase in the level of the liquid in the solution results from a flow of water through the parchment into the solution. The process occurs as a result of a thermodynamic tendency to equalize the sugar concentrations on both sides of the barrier. The parchment permits the passage of water, but hinders that of the sugar, and is said to be semipermeable. Specially treated collodion and cellophane membranes also exhibit this behavior. These membranes are not perfect, and a gradual diffusion of solute molecules into the more dilute solution will occur. Of all artificial membranes, a deposit of cupric ferrocyanide in the pores of a finegrained porcelain most nearly approaches complete semipermeability. The walls of cells in living organisms permit the passage of water and certain solutes, while preventing the passage of other solutes, usually of relatively high molecular weight. These walls act as selectively permeable membranes, and allow osmosis to occur between the interior of the cell and the surrounding media. -

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Conceptual Understanding of Osmosis and Diffusion by Australian First-Year Biology Students
International Journal of Innovation in Science and Mathematics Education, 27(9), 17-33, 2019 Conceptual Understanding of Osmosis and Diffusion by Australian First-year Biology Students Nicole B. Reinkea, Mary Kynna and Ann L. Parkinsona Corresponding author: Nicole B. Reinke ([email protected]) aSchool of Health and Sport Sciences, University of the Sunshine Coast, Sippy Downs QLD 4576, Australia Keywords: biology, osmosis, diffusion, conceptual assessment, misconception Abstract Osmosis and diffusion are essential foundation concepts for first-year biology students as they are a key to understanding much of the biology curriculum. However, mastering these concepts can be challenging due to their interdisciplinary and abstract nature. Even at their simplest level, osmosis and diffusion require the learner to imagine processes they cannot see. In addition, many students begin university with flawed beliefs about these two concepts which will impede learning in related areas. The aim of this study was to explore misconceptions around osmosis and diffusion held by first-year cell biology students at an Australian regional university. The 18- item Osmosis and Diffusion Conceptual Assessment was completed by 767 students. From the results, four key misconceptions were identified: approximately half of the participants believed dissolved substances will eventually settle out of a solution; approximately one quarter thought that water will always reach equal levels; one quarter believed that all things expand and contract with temperature; and nearly one third of students believed molecules only move with the addition of external force. Greater attention to identifying and rectifying common misconceptions when teaching first-year students will improve their conceptual understanding of these concepts and benefit their learning in subsequent science subjects. -

Circle Reverse Osmosis System
CIRCLE REVERSE OSMOSIS SYSTEM KEY FEATURES Water Saving Technology – Patented technology eliminates backpressure The RC100 conforms to common in conventional RO systems making the Circle up to 10 times more NSF/ANSI 42, 53 and efficient than existing products. 58 for the reduction of Saves You Money – Conventional RO systems waste up to 24 gallons of Aesthetic Chlorine, Taste water per every 1 gallon of filtered water produced. The Circle only wastes and Odor, Cyst, VOCs, an average of 2.1 gallons of water per 1 gallon of filtered water produced, Fluoride, Pentavalent Arsenic, Barium, Radium 226/228, Cadmium, Hexavalent saving you water and money over the life of the product!. Chromium, Trivalent Chromium, Lead, RO Filter Auto Flushing – Significantly extends life of RO filter. Copper, Selenium and TDS as verified Chrome Faucet Included – With integrated LED filter change indicator. and substantiated by test data. The RC100 conforms to NSF/ANSI 372 for Space Saving Compact Design – Integrated rapid refill tank means more low lead compliance. space under the sink. SPECIFICATIONS Product Name H2O+ Circle Reverse Osmosis Water Filtration System Model / SKU RC100 Installation Undercounter Sediment Filter, Pre-Carbon Plus Filter, Post Carbon Block Filter (RF-20): 6 months Filters & Lifespan RO Membrane Filter (RF-40): 24 months Tank Capacity 6 Liters (refills fully in less than one hour) Dimensions 9.25” (W) x 16.5” (H) x 13.75” (D) Net weight 14.6 lbs Min/Max Operating Pressure 40 psi – 120 psi (275Kpa – 827Kpa) Min/Max Water Feed Temp 41º F – 95º F (5º C – 35º C) Faucet Flow Rate 0.26 – 0.37 gallons per minute (gpm) at incoming water pressure of 20–100 psi Rated Service Flow 0.07 gallons per minute (gpm) Warranty One Year Warranty PO Box 470085, San Francisco CA, 94147–0085 brondell.com 888-542-3355. -

Solute Concentration: Molality
5/25/2012 Colligative Properties of Solutions . Colligative Properties: • Solution properties that depend on concentration of solute particles, not the identity of particles. Previous example: vapor pressure lowering. Consequences: change in b.p. and f.p. of solution. © 2012 by W. W. Norton & Company Solute Concentration: Molality . Changes in boiling point/freezing point of solutions depends on molality: moles of solute m kg of solvent • Preferred concentration unit for properties involving temperature changes because it is independent of temperature. © 2012 by W. W. Norton & Company 1 5/25/2012 Calculating Molality Starting with: a) Mass of solute and solvent. b) Mass of solute/ volume of solvent. c) Volume of solution. © 2012 by W. W. Norton & Company Sample Exercise 11.8 How many grams of Na2SO4 should be added to 275 mL of water to prepare a 0.750 m solution of Na2SO4? Assume the density of water is 1.000 g/mL. © 2012 by W. W. Norton & Company 2 5/25/2012 Boiling-Point Elevation and Freezing-Point Depression . Boiling Point Elevation (ΔTb): • ΔTb = Kb∙m • Kb = boiling point elevation constant of solvent; m = molality. Freezing Point Depression (ΔTf): • ΔTf = Kf∙m • Kf = freezing-point depression constant; m = molality. © 2012 by W. W. Norton & Company Sample Exercise 11.9 What is the boiling point of seawater if the concentration of ions in seawater is 1.15 m? © 2012 by W. W. Norton & Company 3 5/25/2012 Sample Exercise 11.10 What is the freezing point of radiator fluid prepared by mixing 1.00 L of ethylene glycol (HOCH2CH2OH, density 1.114 g/mL) with 1.00 L of water (density 1.000 g/mL)? The freezing-point-depression constant of water, Kf, is 1.86°C/m. -

Osmosis and Osmoregulation Robert Alpern, M.D
Osmosis and Osmoregulation Robert Alpern, M.D. Southwestern Medical School Water Transport Across Semipermeable Membranes • In a dilute solution, ∆ Τ∆ Jv = Lp ( P - R CS ) • Jv - volume or water flux • Lp - hydraulic conductivity or permeability • ∆P - hydrostatic pressure gradient • R - gas constant • T - absolute temperature (Kelvin) • ∆Cs - solute concentration gradient Osmotic Pressure • If Jv = 0, then ∆ ∆ P = RT Cs van’t Hoff equation ∆Π ∆ = RT Cs Osmotic pressure • ∆Π is not a pressure, but is an expression of a difference in water concentration across a membrane. Osmolality ∆Π Σ ∆ • = RT Cs • Osmolarity - solute particles/liter of water • Osmolality - solute particles/kg of water Σ Osmolality = asCs • Colligative property Pathways for Water Movement • Solubility-diffusion across lipid bilayers • Water pores or channels Concept of Effective Osmoles • Effective osmoles pull water. • Ineffective osmoles are membrane permeant, and do not pull water • Reflection coefficient (σ) - an index of the effectiveness of a solute in generating an osmotic driving force. ∆Π Σ σ ∆ = RT s Cs • Tonicity - the concentration of effective solutes; the ability of a solution to pull water across a biologic membrane. • Example: Ethanol can accumulate in body fluids at sufficiently high concentrations to increase osmolality by 1/3, but it does not cause water movement. Components of Extracellular Fluid Osmolality • The composition of the extracellular fluid is assessed by measuring plasma or serum composition. • Plasma osmolality ~ 290 mOsm/l Na salts 2 x 140 mOsm/l Glucose 5 mOsm/l Urea 5 mOsm/l • Therefore, clinically, physicians frequently refer to the plasma (or serum) Na concentration as an index of extracellular fluid osmolality and tonicity. -

Summary a Plant Is an Integrated System Which: 1
Summary A plant is an integrated system which: 1. Obtains water and nutrients from the soil. 2. Transports them 3. Combines the H2O with CO2 to make sugar. 4. Exports sugar to where it’s needed Today, we’ll start to go over how this occurs Transport in Plants – Outline I.I. PlantPlant waterwater needsneeds II.II. TransportTransport ofof waterwater andand mineralsminerals A.A. FromFrom SoilSoil intointo RootsRoots B.B. FromFrom RootsRoots toto leavesleaves C.C. StomataStomata andand transpirationtranspiration WhyWhy dodo plantsplants needneed soso muchmuch water?water? TheThe importanceimportance ofof waterwater potential,potential, pressure,pressure, solutessolutes andand osmosisosmosis inin movingmoving water…water… Transport in Plants 1.1. AnimalsAnimals havehave circulatorycirculatory systems.systems. 2.2. VascularVascular plantsplants havehave oneone wayway systems.systems. Transport in Plants •• OneOne wayway systems:systems: plantsplants needneed aa lotlot moremore waterwater thanthan samesame sizedsized animals.animals. •• AA sunflowersunflower plantplant “drinks”“drinks” andand “perspires”“perspires” 1717 timestimes asas muchmuch asas aa human,human, perper unitunit ofof mass.mass. Transport of water and minerals in Plants WaterWater isis goodgood forfor plants:plants: 1.1. UsedUsed withwith CO2CO2 inin photosynthesisphotosynthesis toto makemake “food”.“food”. 2.2. TheThe “blood”“blood” ofof plantsplants –– circulationcirculation (used(used toto movemove stuffstuff around).around). 3.3. EvaporativeEvaporative coolingcooling. -

What Is the Difference Between Osmosis and Diffusion?
What is the difference between osmosis and diffusion? Students are often asked to explain the similarities and differences between osmosis and diffusion or to compare and contrast the two forms of transport. To answer the question, you need to know the definitions of osmosis and diffusion and really understand what they mean. Osmosis And Diffusion Definitions Osmosis: Osmosis is the movement of solvent particles across a semipermeable membrane from a dilute solution into a concentrated solution. The solvent moves to dilute the concentrated solution and equalize the concentration on both sides of the membrane. Diffusion: Diffusion is the movement of particles from an area of higher concentration to lower concentration. The overall effect is to equalize concentration throughout the medium. Osmosis And Diffusion Examples Examples of Osmosis: Examples of osmosis include red blood cells swelling up when exposed to fresh water and plant root hairs taking up water. To see an easy demonstration of osmosis, soak gummy candies in water. The gel of the candies acts as a semipermeable membrane. Examples of Diffusion: Examples of diffusion include perfume filling a whole room and the movement of small molecules across a cell membrane. One of the simplest demonstrations of diffusion is adding a drop of food coloring to water. Although other transport processes do occur, diffusion is the key player. Osmosis And Diffusion Similarities Osmosis and diffusion are related processes that display similarities. Both osmosis and diffusion equalize the concentration of two solutions. Both diffusion and osmosis are passive transport processes, which means they do not require any input of extra energy to occur. -

Introduction to Osmosis
DIFFUSION & OSMOSIS EXPLORE LAB SCIENCE WHAT IS DIFFUSION? Diffusion is the net movement of molecules from areas of high concentration to areas of low concentration of that molecule. This random movement, or Brownian motion, is the result of collisions with other molecules in the medium. To see diffusion in action, put a drop of food coloring into a glass of water and see how it behaves. GRADIENTS The movement of particles from high to low concentration is called “moving down the concentration gradient.” Low concentration High concentration EQUILIBRIUM Molecules will continue to diffuse like this until they reach equilibrium, or until there are equal concentrations of that molecule throughout the whole medium. This is why the food coloring particles disperse until the water is the same color all the way through. OSMOSIS Osmosis is a specialized type of diffusion that describes the movement of water molecules. Osmosis occurs when you have membrane with unequal concentrations of water molecules on either side. Water molecules will diffuse across the membrane until equilibrium is reached. SEMIPERMEABLE MEMBRANES When you made your naked eggs, you removed their shell. Now they are surrounded by a semipermeable membrane. What is let across the membrane is determined by the size, shape, or charge of the molecule and the characteristics of the membrane’s pores. The egg membrane is only permeable water in your experiments, so all the changes you see are because of the movement of water across the membrane. NAKED EGG EXPERIMENT If you put your naked egg in But if you put your egg in water, you probably noticed corn syrup or salt water, it that it got bigger. -
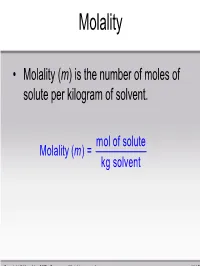
Mol of Solute Molality ( ) = Kg Solvent M
Molality • Molality (m) is the number of moles of solute per kilogram of solvent. mol of solute Molality (m ) = kg solvent Copyright © Houghton Mifflin Company. All rights reserved. 11 | 51 Sample Problem Calculate the molality of a solution of 13.5g of KF dissolved in 250. g of water. mol of solute m = kg solvent 1 m ol KF ()13.5g 58.1 = 0.250 kg = 0.929 m Copyright © Houghton Mifflin Company. All rights reserved. 11 | 52 Mole Fraction • Mole fraction (χ) is the ratio of the number of moles of a substance over the total number of moles of substances in solution. number of moles of i χ = i total number of moles ni = nT Copyright © Houghton Mifflin Company. All rights reserved. 11 | 53 Sample Problem -Conversions between units- • ex) What is the molality of a 0.200 M aluminum nitrate solution (d = 1.012g/mL)? – Work with 1 liter of solution. mass = 1012 g – mass Al(NO3)3 = 0.200 mol × 213.01 g/mol = 42.6 g ; – mass water = 1012 g -43 g = 969 g 0.200mol Molality==0.206 mol / kg 0.969kg Copyright © Houghton Mifflin Company. All rights reserved. 11 | 54 Sample Problem Calculate the mole fraction of 10.0g of NaCl dissolved in 100. g of water. mol of NaCl χ=NaCl mol of NaCl + mol H2 O 1 mol NaCl ()10.0g 58.5g NaCl = 1 m ol NaCl 1 m ol H2 O ()10.0g+ () 100.g H2 O 58.5g NaCl 18.0g H2 O = 0.0299 Copyright © Houghton Mifflin Company. -

Transport of Water and Solutes in Plants
Transport of Water and Solutes in Plants Water and Solute Potential Water potential is the measure of potential energy in water and drives the movement of water through plants. LEARNING OBJECTIVES Describe the water and solute potential in plants Key Points Plants use water potential to transport water to the leaves so that photosynthesis can take place. Water potential is a measure of the potential energy in water as well as the difference between the potential in a given water sample and pure water. Water potential is represented by the equation Ψsystem = Ψtotal = Ψs + Ψp + Ψg + Ψm. Water always moves from the system with a higher water potential to the system with a lower water potential. Solute potential (Ψs) decreases with increasing solute concentration; a decrease in Ψs causes a decrease in the total water potential. The internal water potential of a plant cell is more negative than pure water; this causes water to move from the soil into plant roots via osmosis.. Key Terms solute potential: (osmotic potential) pressure which needs to be applied to a solution to prevent the inward flow of water across a semipermeable membrane transpiration: the loss of water by evaporation in terrestrial plants, especially through the stomata; accompanied by a corresponding uptake from the roots water potential: the potential energy of water per unit volume; designated by ψ Water Potential Plants are phenomenal hydraulic engineers. Using only the basic laws of physics and the simple manipulation of potential energy, plants can move water to the top of a 116-meter-tall tree. Plants can also use hydraulics to generate enough force to split rocks and buckle sidewalks. -

Engineering Aspects of Reverse Osmosis Module Design
Lenntech [email protected] Tel. +31-152-610-900 www.lenntech.com Fax. +31-152-616-289 Engineering Aspects of Reverse Osmosis Module Design Authors: Jon Johnson +, Markus Busch ++ + Research Specialist, Research and Development, Dow Water & Process Solutions ++ Global Desalination Application Specialist, Dow Water & Process Solutions Email:[email protected] Abstract During the half century of development from a laboratory discovery to plants capable of producing up to half a million tons of desalinated seawater per day, Reverse Osmosis (RO) technology has undergone rapid transition. This transition process has caused signification transformation and consolidation in membrane chemistry, module design, and RO plant configuration and operation. From the early days, when cellulose acetate membranes were used in hollow fiber module configuration, technology has transitioned to thin film composite polyamide flat-sheet membranes in a spiral wound configuration. Early elements – about 4-inches in diameter during the early 70s – displayed flow rates approaching 250 L/h and sodium chloride rejection of about 98.5 percent. One of today’s 16-inch diameter elements is capable of delivering 15-30 times more permeate (4000-8000 L/h) with 5 to 8 times less salt passage (hence a rejection rate of 99.7 percent or higher). This paper focuses on the transition process in RO module configuration, and how it helped to achieve these performance improvements. An introduction is provided to the two main module configurations present in the early days, hollow fiber and spiral wound and the convergence to spiral wound designs is described as well. The development and current state of the art of the spiral wound element is then reviewed in more detail, focusing on membrane properties (briefly), membrane sheet placement (sheet length and quantity), the changes in materials used (e.g.