Blomechanlcs of the ACETABULAR LABRUM By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Femur Pelvis HIP JOINT Femoral Head in Acetabulum Acetabular
Anatomy of the Hip Joint Overview The hip joint is one of the largest weight-bearing HIP JOINT joints in the body. This ball-and-socket joint allows the leg to move and rotate while keeping the body Femoral head in stable and balanced. Let's take a closer look at the acetabulum main parts of the hip joint's anatomy. Pelvis Bones Two bones meet at the hip joint, the femur and the pelvis. The femur, commonly called the "thighbone," is the longest and heaviest bone of the body. At the top of the femur, positioned on the femoral neck, is the femoral head. This is the "ball" of the hip joint. The other part of the joint – the Femur "socket" – is found in the pelvis. The pelvis is a bone made of three sections: the ilium, the ischium and the pubis. The socket is located where these three sections fuse. The proper name of the socket is the "acetabulum." The head of the femur fits tightly into this cup-shaped cavity. Articular Cartilage The femoral head and the acetabulum are covered Acetabular with a layer of articular cartilage. This tough, smooth tissue protects the bones. It allows them to labrum glide smoothly against each other as the ball moves in the socket. Soft Tissues Several soft tissue structures work together to hold the femoral head securely in place. The acetabulum is surrounded by a ring of cartilage called the "acetabular labrum." This deepens the socket and helps keep the ball from slipping out of alignment. It also acts as a shock absorber. -

Elbow Checklist
Workbook Musculoskeletal Ultrasound September 26, 2013 Shoulder Checklist Long biceps tendon Patient position: Facing the examiner Shoulder in slight medial rotation; elbow in flexion and supination Plane/ region: Transverse (axial): from a) intraarticular portion to b) myotendinous junction (at level of the pectoralis major tendon). What you will see: Long head of the biceps tendon Supraspinatus tendon Transverse humeral ligament Subscapularis tendon Lesser tuberosity Greater tuberosity Short head of the biceps Long head of the biceps (musculotendinous junction) Humeral shaft Pectoralis major tendon Plane/ region: Logitudinal (sagittal): What you will see: Long head of biceps; fibrillar structure Lesser tuberosity Long head of the biceps tendon Notes: Subscapularis muscle and tendon Patient position: Facing the examiner Shoulder in lateral rotation; elbow in flexion/ supination Plane/ region: longitudinal (axial): full vertical width of tendon. What you will see: Subscapularis muscle, tendon, and insertion Supraspinatus tendon Coracoid process Deltoid Greater tuberosity Lesser tuberosity Notes: Do passive medial/ lateral rotation while examining Plane/ region: Transverse (sagittal): What you will see: Lesser tuberosity Fascicles of subscapularis tendon Supraspinatus tendon Patient position: Lateral to examiner Shoulder in extension and medial rotation Hand on ipsilateral buttock Plane/ region: Longitudinal (oblique sagittal) Identify the intra-articular portion of biceps LH in the transverse plane; then -

Compiled for Lower Limb
Updated: December, 9th, 2020 MSI ANATOMY LAB: STRUCTURE LIST Lower Extremity Lower Extremity Osteology Hip bone Tibia • Greater sciatic notch • Medial condyle • Lesser sciatic notch • Lateral condyle • Obturator foramen • Tibial plateau • Acetabulum o Medial tibial plateau o Lunate surface o Lateral tibial plateau o Acetabular notch o Intercondylar eminence • Ischiopubic ramus o Anterior intercondylar area o Posterior intercondylar area Pubic bone (pubis) • Pectineal line • Tibial tuberosity • Pubic tubercle • Medial malleolus • Body • Superior pubic ramus Patella • Inferior pubic ramus Fibula Ischium • Head • Body • Neck • Ramus • Lateral malleolus • Ischial tuberosity • Ischial spine Foot • Calcaneus Ilium o Calcaneal tuberosity • Iliac fossa o Sustentaculum tali (talar shelf) • Anterior superior iliac spine • Anterior inferior iliac spine • Talus o Head • Posterior superior iliac spine o Neck • Posterior inferior iliac spine • Arcuate line • Navicular • Iliac crest • Cuboid • Body • Cuneiforms: medial, intermediate, and lateral Femur • Metatarsals 1-5 • Greater trochanter • Phalanges 1-5 • Lesser trochanter o Proximal • Head o Middle • Neck o Distal • Linea aspera • L • Lateral condyle • L • Intercondylar fossa (notch) • L • Medial condyle • L • Lateral epicondyle • L • Medial epicondyle • L • Adductor tubercle • L • L • L • L • 1 Updated: December, 9th, 2020 Lab 3: Anterior and Medial Thigh Anterior Thigh Medial thigh General Structures Muscles • Fascia lata • Adductor longus m. • Anterior compartment • Adductor brevis m. • Medial compartment • Adductor magnus m. • Great saphenous vein o Adductor hiatus • Femoral sheath o Compartments and contents • Pectineus m. o Femoral canal and ring • Gracilis m. Muscles & Associated Tendons Nerves • Tensor fasciae lata • Obturator nerve • Iliotibial tract (band) • Femoral triangle: Boundaries Vessels o Inguinal ligament • Obturator artery o Sartorius m. • Femoral artery o Adductor longus m. -

Acetabular Labral Tears: Resection Vs
Acetabular Labral Tears: Resection vs. Repair In the past decade, significant advances have been made in the diagnosis and treatment of non-arthritic intra-articular hip pathologies, including acetabular labral tears. Tears of the acetabular labrum have been identified as a source of hip pain and mechanical symptoms, and as a possible instigator of premature hip degeneration. Arthroscopic management of labral tears has evolved from simple resection of the torn labral portion to advanced repair techniques for tears Mara L. Schenker, MD1 associated with large bony deformities. While arthroscopic technology has evolved to allow the labrum to be repaired, Marc J. Philippon, MD2 scientific evidence demonstrating the benefits of labral repair over resection have lagged. 1 Department of Orthopaedic Surgery, University of Pennsylvania, Philadelphia, PA In the past decade, significant advances have quicker rate of cartilage consolidation in the 2 Steadman Philippon Research Institute, been made in the diagnosis and treatment of absence of a labrum. They further demonstrated Vail, CO non-arthritic intra-articular hip pathologies, that resection of the labrum causes the femoral including acetabular labral tears. Tears of the head to lateralize, shifting the load bearing surface acetabular labrum have been identified as a of the joint shifts to the acetabular rim, thereby source of hip pain and mechanical symptoms, causing increases in femoroacetabular contact and as a possible instigator of premature hip pressures6. Although some have suggested degeneration1. Arthroscopic management of that labral resection may lead to premature labral tears has evolved from simple resection osteoarthritis, one in vivo study failed to show the of the torn labral portion to advanced repair relationship at 24 months after labral resection7. -

Acetabular Labral Tears with Underlying Chondromalacia: a Possible Association with High-Level Running
Acetabular Labral Tears With Underlying Chondromalacia: A Possible Association With High-Level Running Carlos A. Guanche, M.D., and Robby S. Sikka, B.A. Purpose: The use of hip arthroscopy has helped delineate intra-articular pathology and has enabled clinicians to further elucidate the factors responsible for injuries, such as running. The subtle development of degenerative changes may be a result of repetitive impact loading associated with this sport. This study presents a population of runners with common pathologic acetabular changes. Type of Study: Case series. Methods: Eight high-level runners with an average age of 36 years (range, 19 to 45 years) were seen for complaints of increasing hip pain with running without any history of macrotrauma. All of the patients had either run several marathons (4), were triathletes (1), Olympic middle distance runners (1), or had run more than 10 miles per week for longer than 5 years (2). Plain radiographic analysis revealed no degenerative changes and an average center-edge (CE) angle of 36.7° (range, 28° to 44°). Results: All patients underwent hip arthroscopy with labral debridement. In 6 patients (75%), a chondral injury of the acetabular cartilage underlying the labral tear was noted. In addition, 3 patients had ligamentum teres disruptions. Conclusions: It is possible that the development of these tears is the result of subtle instability, which may be exacerbated by running, eventually leading to labral tearing and possible ligamentum teres disruption. While perhaps con- currently, subtle acetabular dysplasia may play a role. Although this study does not confirm an association between running and the development of labral tears or chondral lesions in the hip, it certainly questions whether there is an injury pattern common to this population, a “runner’s hip.” Level of Evidence: Level IV. -

Hip Arthroscopy for Acetabular Labral Tears
Hip Arthroscopy for Acetabular Labral Tears Laith A. Farjo, M.D., James M. Glick, M.D., and Thomas G. Sampson, M.D. Summary: The purpose of this study is to better understand the history, physical examination, imaging, and outcome of arthroscopic debridement of acetabular labral tears. We performed a review of all 290 patients who underwent hip arthroscopy at our institution to identify those who have undergone arthroscopic debridement of an acetabular labral tear. Patients were assessed at follow-up by a physician visit or telephone interview and questioned as to pain, mechanical symptoms, activity level, work status, sports ability, and performance of activities of daily living. Patients were followed-up for a minimum of 1 year or until they underwent total hip arthroplasty (THA). All 28 patients meeting the study criteria were available for follow-up (mean age, 41 years; range, 14 to 70 years) at an average of 34 months after surgery (range, 13 to 100 months). Average duration of symptoms before arthroscopy was 25 months. Eighteen (64%) patients were noted to have mechanical symptoms such as clicking or locking. Ten patients were noted to have a specific inciting event that initiated their symptoms. Magnetic resonance imaging identified the labral tear in 5 of 21 (24%) cases; arthrography identified the tear in 1 of 8 (13%). Of the 28 tears identified, there were 12 radial flap, 5 degenerative, 5 bucket handle, 3 horizontal cleavage, and 3 peripheral longitudinal tears. Seventeen were located anteriorly, 7 were located posteriorly, and 4 were located superiorly. Patients were stratified into two groups based on the presence of significant joint arthritis on radiographs. -

Microinstability of the Hip: a Previously Unrecognized Pathology
Original article Microinstability of the hip: a previously unrecognized pathology Ioanna Bolia 1 The role of the hip joint capsule has gained par - Jorge Chahla 1 ticular research interest during the last years, and Renato Locks 1 its repair or reconstruction during hip arthro - Karen Briggs 1 scopy is considered necessary in order to avoid Marc J. Philippon 1,2 iatrogenic hip microinstability. Various capsular closure/plication techniques have been devel - oped towards this direction with encouraging re - 1 Steadman Philippon Research Institute, Colorado, USA sults. 2 The Steadman Clinic, Colorado, USA Level of evidence: V. KEY WORDS: hip arthroscopy, hip microinstability, hip Corresponding author: dysplasia. Marc J. Philippon, MD Steadman Philippon Research Institute The Steadman Clinic Introduction 181 West Meadow Drive, Suite 400 Vail, Colorado 81657, USA The native hip is a particularly constrained joint with a E-mail: [email protected] powerful suction seal that is imperative for optimal 1,2 function of the joint . The hip capsule is one of the most important static stabilizers of the hip joint 3 and Summary disruption or debridement of the capsule during hip arthroscopy is a potential contributor to postoperative Background : Hip microinstability is an estab - iatrogenic hip instability. Therefore, hip surgeons must lished diagnosis; however, its occurrence is still be thoughtful of hip capsule management as hip debated by many physicians. Diagnosis of hip mi - arthroscopic procedures are increasing exponentially 4. croinstability is often challenging, due to a lack of Unlike other joints in the anatomy, hip instability is specific signs or symptoms, and patients may re - generally defined as extra-physiologic hip motion that main undiagnosed for long periods. -

Effect of Acetabular Labral Tears on Proprioceptive Parameters of the Human Hip Joint HIP CENTER David C
Effect of Acetabular Labral Tears on Proprioceptive Parameters of the Human Hip Joint HIP CENTER David C. Holst,,, Allston J. Stubbs MD, Martin L. Tanaka PhD 1. Introduction (dominant or non-dominant for controls and pathologic or 5. Conclusions Acetabular labral cartilage tears are a common cause of hip pain normal for patients) was randomized using a coin toss. A Based on our limited data set, two concepts can be drawn from our in younger, athletic individuals and are associated with the total of 7 trials were completed for each side, with the first early results. First, computer based balance analysis (a development of hip joint arthritis. The pain of labral tears trial on each leg counting as “practice,” and not included in noninvasive test) can successfully differentiate normal subjects and 1 negatively affects patients’ daily activities and athletic goals. final analyses. The force plate measured Center of Pressure symptomatic hip patients based on single leg balance. Second, These labral tears are difficult to diagnose despite the (COP)deviations(Figure2),andthesedatawererecorded patients with labral tears appear to have worse balance in the availability of advanced technology such as MRI scanning. Due to and subsequently processed using custom MATLAB code. affected leg than the unaffected leg. In conclusion, we limited diagnostic tests, arthroscopic hip surgery is often used to Measurements included the area of an ellipse that enveloped recommend continuation of this projectasameanstoestablish confirm cartilage tears of the hip joint. As surgery is invasive, 95% of the data points (COP_EA, measured in mm2)andthe proprioception balance testing as a noninvasive means to assess for improved non-invasive diagnostic tools for this condition are path length of the COP (measured in mm). -

Arthroscopic Management of Labral Tears in the Hip
This is an enhanced PDF from The Journal of Bone and Joint Surgery The PDF of the article you requested follows this cover page. Arthroscopic Management of Labral Tears in the Hip Michael K. Shindle, James E. Voos, Shane J. Nho, Benton E. Heyworth and Bryan T. Kelly J Bone Joint Surg Am. 2008;90:2-19. doi:10.2106/JBJS.H.00686 This information is current as of September 26, 2010 Reprints and Permissions Click here to order reprints or request permission to use material from this article, or locate the article citation on jbjs.org and click on the [Reprints and Permissions] link. Publisher Information The Journal of Bone and Joint Surgery 20 Pickering Street, Needham, MA 02492-3157 www.jbjs.org Shindle.fm Page 2 Wednesday, October 15, 2008 12:17 PM 2 COPYRIGHT © 2008 BY THE JOURNAL OF BONE AND JOINT SURGERY, INCORPORATED Arthroscopic Management of Labral Tears in the Hip By Michael K. Shindle, MD, James E. Voos, MD, Shane J. Nho, MD, Benton E. Heyworth, MD, and Bryan T. Kelly, MD Introduction similar to the healing potential of the menisci in the knee, ver the last decade, the diagnosis and arthroscopic which is greatest at the periphery, the healing potential of the management of labral tears of the hip in the young labrum is greatest at the peripheral capsulolabral junction5-8. Oathletic population has evolved substantially due to The labrum has an important sealing function in the improvements in clinical examination, diagnostic tools, sur- hip. It plays a role in limiting the expression of fluid from the gical techniques, and flexible instrumentation in hip arthros- joint space and also helps contain the femoral head at extreme copy. -
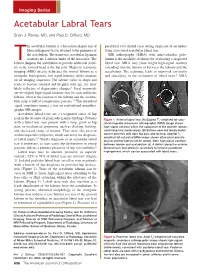
Acetabular Labral Tears
Imaging Series Acetabular Labral Tears Brian J. Parker, MD, and Paul D. Clifford, MD he acetabular labrum is a horseshoe-shaped rim of paralabral cyst should raise strong suspicion of an under- fibrocartilaginous tissue attached to the perimeter of lying associated acetabular labral tear. the acetabulum. The transverse acetabular ligament MR arthrography (MRA) with intra-articular gado- connects the 2 inferior limbs of the horseshoe. The linium is the modality of choice for evaluating a suspected Tlabrum deepens the acetabulum to provide additional stabil- labral tear. MRA may show bright high-signal contrast ity to the femoral head at the hip joint. Magnetic resonance extending into the labrum or between the labrum and the imaging (MRI) directly delineates the normal labrum as a acetabulum. The technique leads to improved sensitivity triangular, homogenous, low-signal-intensity (dark) structure and specificity in the evaluation of labral tears.1 MRA on all imaging sequences. The labrum varies in shape and tends to become rounded and irregular with age, the latter likely reflective of degenerative changes.1 Focal intermedi- ate–to–slightly high–signal intensity may be seen within the labrum, often at the junction of the labrum and the acetabu- lum, in up to half of asymptomatic patients.1,2 This intralabral signal sometimes mimics a tear on conventional nonarthro- graphic MR images. Acetabular labral tears are a recognized cause of hip A B pain in the absence of plain radiographic findings. Patients Figure 1. Anterior labral tear. (A) Sagittal T1-weighted fat-satu- with a labral tear may present with a limp, groin or hip rated magnetic resonance arthrographic (MRA) image shows pain, or mechanical symptoms, such as clicking, locking, high-signal contrast within the substance of the anterior labrum, and decreased range of motion. -

Prevalence of Labral Tears
Department of Rehabilitation Services Physical Therapy Standard of Care: Acetabular Labral Tears Non-Operative Treatment Case Type / Diagnosis: Acetabular Labral Tear ICD 10 Codes: S73.191D Tear of acetabulum labrum, right, subsequent encounter primary S73.192D Tear of acetabulum labrum, left, subsequent encounter primary M16.9 Labral tear of hip, degenerative M76.891 Enthesopathy of hip, right M76.892 Enthesopathy of hip, left M25.551 Hip pain, right M25.552 Hip pain, left M25.551 Pain in joint, pelvic region and thigh, right M25.551 Pain in joint, pelvic region and thigh, left M76.891 Femoroacetabular Impingement, right M76.892 Femoroacetabular Impingement, right Preferred Therapy Practice Pattern(s) Musculoskeletal - impaired joint mobility, motor function, muscle performance, and range of motion associated with connective tissue dysfunction The acetabular labrum (labrum) is a structure akin to the meniscus of the knee and the glenoid labrum of the shoulder1. The labrum is a 2-3 mm thick fibrocartilaginous structure, which covers the rim of the acetabulum and serves multiple functions including: stability, joint integrity, and lubrication.2 It enhances stability by structurally deepening the acetabulum. A fluid seal is created by the labrum which contains the synovial fluid within the articular cartilage and maintains lubrication3. The labrum decreases force distribution at the hip joint by preventing direct contact between the femoral head and the acetabulum.2,4 A study by Ferguson that analyzed the difference in joint stress without a labrum suggested the hip contact stress increases by 92%3. The labrum can be torn secondary to high forces at the hip joint, which can lead to joint instability with consequent labral fraying as well as chondral degeneration.4,5 The labrum also provides proprioceptive feedback through free nerve endings which, in a pathological joint, can be a source of pain6,7. -

Femoroacetabular Impingement (FAI) - Patient Information
Femoroacetabular impingement (FAI) - Patient information Femoroacetabular impingement (FAI) What is femoroacetabular impingement? Femoroacetabular impingement (FAI) is a condition in which there is abnormal contact (impingement) between the rim of the acetabulum (hip joint socket) and femoral head-neck junction (the bone just below the ball part of the thigh bone), on movement of the hip. The most common movement that brings on pain is hip flexion (knee towards chest). Patients will experience pain, usually in the groin, but sometimes further down the front of the thigh, side or back of the hip. There may be episodes of clicking in the hip, or the sensation that it is coming out of joint. Certain activities, particularly those which involve hip flexion (e.g. football, dancing, ballet, and aerobics) will make the pain worse. Patients often find that sitting for a prolonged period of time, e.g. a long car journey, will bring on groin pain and they often struggle to move into a more comfortable position. Diagram of the hip joint Femoroacetabular impingement (FAI) – July 2019 Patient information - Femoroacetabular impingement (FAI) How does FAI occur? The mechanism of FAI was only described as recently as 2005. It is important to understand that all hips will impinge in people showing no symptoms, but only at the extremes of movement. The difference between a normal hip and one with FAI is that the hip impinges much earlier in a patient with FAI. This occurs because of subtle differences in the anatomy of the hip joint. Essentially this difference is a surplus of bone, either on the edge of the acetabulum or on the femoral head-neck junction or both.