D Y E S C L a S S I F I E I B Y I N T E R M E D I a T E ! Dyes Tabularly Arranged Under Each Intermediate, with Statistical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Innovation Spotlight the Sustainable Revolution in Jeans Manufacture
Innovation Spotlight ADVANCED DENIM The sustainable revolution Issue: Spring 2012 in jeans manufacture Innovative dyeing process spares the environment, offers greater color variety and higher quality Whether elegant or artificially aged, worn with a jacket or a T-shirt: jeans go well with almost anything. They are simultaneously a lifestyle statement, worldwide cult classic and long-selling fashion garment – with no end to the success story in sight. The statistics tell us that a US American has eight pairs of jeans, while a European comes a close second with five to six pairs. The immense number of almost two billion pairs of jeans are produced each year, claiming about 10 percent of the worldwide cotton harvest. The conventional indigo dyeing process, however, is environmentally polluting, and so Clariant has now developed, under its innovative Advanced Denim concept, a groundbreaking new dyeing process adapted to current needs that operates completely without indigo. It also needs much less water and energy, greatly reduces cotton waste and produces no effluents. Furthermore it offers a greater variety of colors, better color quality and new fashion effects. Experts are convinced: Advanced Denim will revolutionize jeans production. Denim is the name given to the typical, tough jeans material which is produced from cotton yarn and in the conventional process is dyed blue with indigo. In its natural agglomerated CLARIANT INTERNATIONAL LTD form, this dye isn’t soluble in water. The dye molecules first have to be separated before BUSINESS UNIT TEXTILE CHEMICALS Rothausstrasse 61 dyeing – this is done by reduction using the strong reducing agent sodium hydrosulfite. -

The International Pharmacopoeia
The International Pharmacopoeia THIRD EDITION Pharmacopoea internationalis Editio tertia Volume 4 Tests, methods, and general requirements Quality specifications for pharmaceutical substances, excipients, and dosage forms World Health Organization Geneva 1994 WHO Library Cataloguing in Publication Data The International Pharmacopoeia.- 3rd ed. Contents: v. 4. Tests, methods, and general requirements 1. Drugs -analysis 2. Drugs -standards ISBN 92 4 154462 7 (NLM Classification: QV 25) The World Health Organization welcomes requests for permission to reproduce or translate its publica- tions, in part or in full. Applications and enquiries should be addressed to the Of£ice of Publications, World Health Organization, Geneva, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available. O World Health Organization, 1994 Publications of the World Health Organization enjoy copyright protection in accordance with the provi- sions of Protocol 2 of the Universal Copyright Convention. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers' products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distin- guished by initial capital letters. -

United States Patent 19 11 Patent Number: 5,749,923 Olip Et Al
USOO5749923A United States Patent 19 11 Patent Number: 5,749,923 Olip et al. 45) Date of Patent: May 12, 1998 54 METHOD FOR BLEACHING DENIM OTHER PUBLICATIONS TEXT LE MATERIAL Federal Law Gazette, No. 612, Sep. 24, 1992, "Limitation of Waste Water Emissions from Textile Finishing and Process 75 Inventors: Winzenz Olip. Schächtestrasse, ing Plants". Austria; Norbert Steiner. Upper Saddle Peter, M., et al. Grundlagen der Textilveredelung Basics of River, N.J. Textile Finishing, 13th ed., Deutscher Fachverlag, 1989, pp. 73 Assignee: Degussa Aktiengelschaft, Frankfurt am 80 to 81. (Month Unknown). Main, Germany Derwent Acc. No. 80-24863C, 1980 (month unknown). Derwent Acc. No. 86-268586, 1986 (Month Unknown). Derwent Acc. No. 89-155166, 1989 (Month Unknown). 21 Appl. No.: 651,785 Das, T.K., et al., “Thiourea Dioxide: A Powerful And Safe 22 Filed: May 24, 1996 Reducing Agent For Textile Applications”. Colourage, vol. 31, No. 26, 1984, pp. 15-20. (Month Unknown). Related U.S. Application Data Weiss, M., "Thiourea Dioxide: A Safe AlternativeTo Hydro sulfite Reduction”. Part 1. American Dyestuff Reporter; vol. 63 Continuation of Ser. No. 347,146, Nov. 22, 1994, Pat. No. 67. No. 8, Aug. 1978, pp. 35-38. 5,549,715. Weiss, M., "Thiourea Dioxide: A Safe Alternative to Hydro 30 Foreign Application Priority Data sulfite Reduction, Part II", American Dyestuff Reporter, vol. 67, No. 9, Sep. 1978, pp. 72-74. Nov. 23, 1993 AT Austria .............................. AT 2378/93 Primary Examiner-Alan Diamond (51 int. Cl. ... D06L 3/10 Attorney, Agent, or Firm-Spencer & Frank 52 U.S. Cl. ........................... 8/102; 8/107; 8/110; 8/111; 510/302; 510/303; 5101494; 510/367; 510/370; 57 ABSTRACT 510/470 Amethod for chlorine-free bleaching of denim textile mate 58 Field of Search ............................... -

Dyes & Pigments
Dyes & Pigments with forecasts to 2005 and 2010 New study finds: • The US market for dyes and organic pigments (organic colorants) is expected to increase 2.8 percent per year to $3.1 billion in 2005, with volume over the same period forecast to reach 675 million pounds • Positive growth opportunities are to be found in the rapidly growing market for dyes used in digital printing inks and high-tech imaging • The six leading suppliers -- Ciba Specialty Chemicals, DyStar, Clariant, Sun Chemical, Bayer and BASF -- accounted for nearly two-thirds of the total market in 2000 Freedonia Industry Study #1439 Study Publication Date: June 2001 Price: $3,700 Dyes & Organic Pigments Pages: 237 Dyes & Organic Pigments, a new study from The Freedonia Group, provides you with an in-depth analysis of major trends in the industry and the outlook for product seg- ments and major markets -- critical information to help you with strategic planning. This brochure gives you an indication of the scope, depth and value of Freedonia's new study, Dyes & Organic Pigments. Ordering information is included on the back page of the brochure. Brochure Table of Contents Study Highlights ............................................................................... 2 Table of Contents and List of Tables and Charts ............................. 4 Sample Pages and Sample Tables from: Market Environment .................................................... 6 Products ....................................................................... 7 Markets....................................................................... -

Chromatographic and Mass Spectral Analyses of Oligosaccharides and Indigo Dye Extracted from Cotton Textiles with Manova and Ano
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2008 Chromatographic And Mass Spectral Analyses Of Oligosaccharides And Indigo Dye Extracted From Cotton Textiles With Manova And Ano Jessica Frisch University of Central Florida Part of the Chemistry Commons, and the Forensic Science and Technology Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Masters Thesis (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Frisch, Jessica, "Chromatographic And Mass Spectral Analyses Of Oligosaccharides And Indigo Dye Extracted From Cotton Textiles With Manova And Ano" (2008). Electronic Theses and Dissertations, 2004-2019. 3625. https://stars.library.ucf.edu/etd/3625 CHROMATOGRAPHIC AND MASS SPECTRAL ANALYSES OF OLIGOSACCHARIDES AND INDIGO DYE EXTRACTED FROM COTTON TEXTILES WITH MANOVA AND ANOVA STATISTICAL DATA ANALYSES by JESSICA LYNNE FRISCH B.S. University of Central Florida, 2005 A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in the Department of Chemistry in the College of Sciences at the University of Central Florida Orlando, Florida Spring Term 2008 © 2008 Jessica Lynne Frisch ii ABSTRACT Research was conducted on thirteen 100% cotton denim samples using an acid wash, established by Murray, to extract oligosaccharides from the cellulosic material. The oligosaccharide ion + + + groups ([M+H] , [M+NH4] , and [M-OH] ) for molecules with degrees of polymerization between two and seven (DP2-DP7) were analyzed using liquid chromatography coupled to mass spectrometry with an electrospray ionization interface (LC-ESI-MS). -

What Is Sera® Fil SBS Based On?
REVISTADE ORGANO OFICIAL DE LA ASOCIACIÓN ESPAÑOLA DE QUÍMICOS Y COLORISTAS TEXTILES Nº214 / NOVIEMBRE 2015 www.aeqct.org Miembro de la FIAQCT Miembro Adherido a la FLAQT Con la colaboración de TEXFOR Les esperamos en / NOVIEMBRE 2015 Nº214 - AEQCT SUMALLA S.L. Plaza Joaquin Folguera, 5, Entlo. 6ª | |08022 - Barcelona - España Tel: (+34) 93 2 09 99 57 | Fax: (+34) 93 2 02 03 90 [email protected] www.gruposumalla.com Denim 2015_Revista_FullPage 20.05.15 11:29 Seite 1 Competence in Denim Finishing Excellence in Dyeing & Finishing ITMA Hall 10 / E101 Aguilar & Pineda Asociados, S.L. Tel.: 0034-93-4876667 C/Mallorca, 279, principal 3° Fax: 0034-93-4880375 08037 Barcelona [email protected] A. Monforts Textilmaschinen GmbH & Co. KG Germany | A Member of CHTC Fong’s Industries www.monforts.com Sumario Revista de Química e Industria Textil • Núm. 214 • Noviembre 2015 5 Editorial 7 Determinación rápida de la degradación de lanas y pelos. F. Marsal 12 Estudio de la capacidad de desintegración de agrotextiles obtenidos a partir de fibras biodegra- dables. M. Ferrándiz, L.Capablanca, D.García, E. Bou- Belda, O. Gutiérrez 20 Los materiales augéticos y su potencial en el sector textil. E. Bou-Belda, P. Díaz-García, I. Montava, M. Bonet-Aracil 27 Denim Book by Archroma. Capítulos 5 y 6. La Revista de Química e Industria Textil con cuatro números anuales, pretende informar sobre las 44 ¿Cómo resolvería Sherlock Holmes los problemas novedades tanto nacionales como internacionales relacionadas con el sector textil. Se distribuye de en la empresal. forma gratuita a los asociados de la AEQCT. -

Lexique-Anglais-Francais.Pdf
Description Terme anglais Terme français Description française anglaise Laine entière, pure, non 100% wool Only wool, pure. laine 100% mélangée. 4 box loom métier à 4 boîtes métier à tisser à 4-box loom quatre boîtes above the knee au dessus du genou Action d’user. Enlèvement The act of polishing, abrasion abrasion par raclage superficiel de grinding. certains tissus. The ability of a fabric to withstand loss of appearance, utility, La solidité d’un tissu, abrasion résistance à pile or surface through comment il conserve ou perd resistance l’abrasion the destructive action ses propriétés face à l’usure. of surface wear and rubbing. A mechanical instrument that tests a Instrument pour mesurer la fabric’s resistance to abrasion tester abrasimètre résistance à l’usure et au the destructive actions temps des tissus. of surface wear and rubbing. coton hydrophile absorbant cotton (ouate) Qui absorbe les liquides, les absorbent Able to absorb. absorbant gaz, les radiations. absorbent cotton- ouate hydrophile wool This fabric don’t permit Tissu qui n’est pas absorbent fabric the passage of a fluid tissu absorbant imperméable, qui absorbe through its substance. les liquides. Qui ne représente pas le monde sensible (réel ou Having only intrinsic imaginaire); qui utilise la abstract form with little or no abstrait (dessin) matière, la ligne et la pictural representation. couleur pour elles-mêmes. Dessin sans référence à la réalité concrète. abstract design dessin abstrait Motif sans référence à la Motif without pictural abstract motif motif abstrait réalité concrète. Motif non- representation. figuratif. Substance qui accélère une réaction. Substance chimique Something that utilisée pour augmenter la accelerant accélérateur accelerate a reaction. -

Biomolecules - Biochimiques - Standards
BIOMOLECULES - BIOCHIMIQUES - STANDARDS H Tél. : 33 (0)4 70 03 88 55 I Hotline : 33 (0)4 70 03 73 06 I E-mail : [email protected] I Fax : 33 (0)4 70 03 82 60 BIOMOLECULES - BIOCHIMIQUES - STANDARDS BIOMOLECULES - BIOCHIMIQUES - STANDARDS Standards de Dioxyde de Carbone H.93 Standards et réactif pour analyseurs de Chlorure H.94 Standards Couleur Hazen H.94 Standards Redox H.94 Standards d'Electrodes sélectives d'ions H.95 Solutions d'ajustage de force ionique H.95 Standards de chromatographie ionique H.96-H.97 Standards pour ICP H.98-H.106 Standards pour Photométrie de flamme H.107-H.108 Organiques H.109-H.123 Composés organiques volatils (COV) H.109 Biomolécules & Composés actifs H.2-H.36 Standards Mélangés de Composés Introduction H.2 Organiques Volatils H.110-H.111 Peptides H.3-H.4 Phénols H.112-H.113 Peptides bloquants pour anticorps H.3 Hydrocarbures aromatiques Peptides (bioactifs, aptamères, viraux…) H.3-H.4 polycycliques (HAP) H.114-H.115 Standards de Carbone Organique Total (COT) H.116 Protéines H.5-H.28 Pesticides H.117-H.118 Kinases H.5-H.13 Standards Lipidiques Avanti Polar Lipids H.119-H.121 Phosphatases Actives H.14 Standards Lipidiques Larodan H.122 Phosphodiestérases Actives H.14 Standards individuels - Contrôles - Histone Déacétylases Actives H.15 Calibrateurs pour hématologie H.123 Acetyltransférases Actives H.15 Acétyl/Methyltransférases Inactives H.15 Couleurs H.124-H.126 Autres enzymes H.16 Standards de couleurs H.124-H.126 Substrats Enzymatiques H.17-H.18 Spectrophotométrie H.127-H.130 Marqueurs CD H.19 Standards -

The Use of Glucose As Ecological Reducing Agent for Sulphur Dyes: Optimization of Experimental Conditions
European Scientific Journal June 2014 edition vol.10, No.18 ISSN: 1857 – 7881 (Print) e - ISSN 1857- 7431 THE USE OF GLUCOSE AS ECOLOGICAL REDUCING AGENT FOR SULPHUR DYES: OPTIMIZATION OF EXPERIMENTAL CONDITIONS Romdhani Zouhaier Dhouib Sofiène Sakli Faouzi Laboratory of Textile Research, ISET of Ksar, Hellal, Monastir, Tunisia Abstract At the present time, dyeing of textile material requires the use of various auxiliaries. These used agents have adverse effects on the environment. In general, for the dyeing with sulphur dyes, a reducing agent is used to transform the dye molecule to a water soluble leuco form that can diffuse into the fibre. In this study, three reducing agents were used : the sodium sulphide, sodium dithionite and glucose as an environmentally friendly reducer. To compare their effect on the reduction of the sullphur dyestuff, the redox potential, pH and the colour yield (K/S) were measured according to the concentration of reducing agent and caustic soda concentration. Results of leuco sulphur black B reduction by the different reducing agents were evaluated by measuring the colour yield (K/S) and the brightness variation after the washing process. The obtained results showed that glucose can offer some scientifically results similar to those done with sodium sulphide which can give for the reduction of sulphur dyestuff Keywords: Sulphur dye, Dyeing, Reduction, Reducing Agent, Glucose Introduction Cellulosic substrates especially cotton, rayon, and paper, are very hydrophilic and, therefore, require hydrophilic soluble dyes for their coloration from a dye bath. Current dyes designed for cellulosic polymers are direct, vat, sulfur, and reactive dyes. Sulphur dyes are one of the less costs of all dye classes used on cellulosic fibres and their blends (Senior & Clarke, 1986). -

ABSTRACT YI, DING. a Comparison of Mordant and Natural Dyes In
ABSTRACT YI, DING. A Comparison of Mordant and Natural Dyes in Dyeing Cotton Fabrics. (Under the direction of Dr. Harold S. Freeman and Dr. Peter J. Hauser). Mordant dyes constitute a class of synthetic colorants that are applied to textile fibers mainly through the aid of transition metal ions such as Cr3+. The resultant dye-metal complexes are key to the fastness properties produced on wool. In the same way, most natural dyes require the use of a mordant to have coloring power on textiles such as cotton. Unlike mordant dyes on wool, the fastness properties of natural dyes on cotton are generally quite low. Consequently, they have largely been replaced by synthetic dyes that are more cost effective, brighter, and more durable under end-use applications. Interest in green technologies has led to renewed consideration of natural dyes for textile coloration because they are biodegradable and do not involve manufacturing processes requiring genotoxic aromatic amines. Interestingly, such a shift would bring dyeing technology full circle to a family of colorants lacking the vibrancy and technical properties of most synthetic dyes. However, similarities between the dyeing method for mordant and natural dyes brought to mind the potential for reducing the level of synthetic dyes used commercially by combining suitable dyes from these two classes for dyeing textiles. This thesis research was devoted to stage 1 of this idea, namely the evaluation of mordant dyes as colorants for cotton fabric. With this in mind, dyes such as Mordant Blue 13, Mordant Brown 40, Mordant Orange 6 and Mordant Yellow 8 were applied to cotton using various dyeing times, temperatures, dye bath concentrations and mordants. -

K/S Shade Values by Wavelength for Typical 3.0 Gm/Lit Indigo Dye Set-Up
ABSTRACT HOLBERT, JR., RICHARD MOORE. Empirical and Theoretical Indigo Dye Models Derived from Observational Studies of Production Scale Chain Rope Indigo Dye Ranges. (Under the direction of Peter Hauser, Warren Jasper, Jon Rust, and Richard Gould.) An observational study of production scale chain rope indigo dye ranges was conducted using 100% cotton open end spun yarns to confirm previously published dye trends, investigate the effects of dye range speed, and develop dye prediction models. To achieve these objectives, several milestones were identified and systematically addressed. A comprehensive laboratory preparation method was developed to ensure consistent yarn preparation. Equilibrium sorption experiments were conducted to determine the functional relationship between dye bath concentration and pH to indigo dye uptake in the cotton yarn. Additionally, the resulting shade from equilibrium sorption data was expanded to create an innovative method of quantitatively characterizing indigo penetration level of non-uniformly dyed yarns. The following dye range set-up conditions were recorded for each observational point: yarn count, number of dips, dye range speed, dwell length, nip pressure, dye bath indigo concentration, dye bath pH, dye bath reduction potential, and oxidation time. All observations were conducted after the dye range had been running for several hours and no feed rate adjustments were required. Later the following measurements were taken to determine each response variable state: total percent chemical on weight of yarn, percent of fixed indigo on weight of yarn, and Integ shade value. Analysis of data from the observational study confirmed most previously published dye trends relating to dye uptake, shade, and penetration level. Notably, the percent indigo on weight of yarn as a function of dye bath pH was not confirmed. -
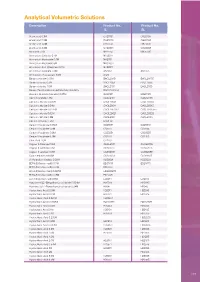
Analytical Volumetric Solutions
Analytical Volumetric Solutions Description Product No. Product No. 1L 5L Acetic acid 0.1M CH20101 CH20105 Acetic acid 0.5M CH20051 CH20055 Acetic acid 1.0M CH21001 CH21005 Acetic acid 2.0M CH22001 CH22005 Ammonia 0.1M NH20101 NH20105 Ammonium Chloride 0.1M NHCl011 Ammonium Hydroxide 0.5M NH2051 Ammonium Hydroxide 6M NH32601 Ammonium Iron (II)Sulphate 0.1M NHS2011 Ammonium sulphate 0.5M AS2051 AS2055 Ammonium Thiocyanate 1.0M AT21F Barium chloride 0.05M BACL20051 BACL20055 Barium chloride 0.5M BACL2051 BACL2055 Barium chloride 1.0M BACL2101 BACL2105 Barium Perchlorate 0.005M Alcoholic Solution BACLO200051 Bromine (Bromate/bromide) 0.05M BR20101 BR20105 Calcium acetate 1.0M CAAC2101 CAAC2105 Calcium chloride 0.005M CACL20051 CACL20055 Calcium chloride 0.01M CACL20011 CACL20015 Calcium chloride 0.0125M CACL2001251 CACL2001255 Calcium chloride 0.02M CACL20021 CACL20025 Calcium chloride 0.5M CACL2051 CACL2055 Calcium Chloride 1.0M CACL101 Cerium IV sulphate 0.05M CS20051 CS20055 Cerium IV sulphate 0.1M CS2011 CS2015 Cerium IV sulphate 0.2M CS20251 CS20255 Cerium IV sulphate 1.0M CS2101 CS2105 Citric Acid 1.0M CH2101 Copper II Chloride 0.5M CUCL2051 CUCL2055 Copper II sulphate 0.1M CUS02011 CUS02015 Copper II sulphate 0.5M CUS02051 CUS02055 Cupric solution 0.168M CU201681 CU201685 Di-Potassium Oxalate 0.05M KO20051 KO20055 EDTA (DiSodium salt) 0.01M ED20011 ED20015 EDTA (DiSodium salt) 0.02M ED20021 EDTA (DiSodium Salt) 0.027M EDB200271 EDTA (DiSodium salt) 0.05M ED20051 EDTA (DiSodium salt) 0.1M ED2011 ED2015 Hyamine 1622 -Benzethonium chloride