Lecture 2 (Pdf)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Arxiv:1904.10318V1 [Nucl-Th] 20 Apr 2019 Ucinltheory](https://docslib.b-cdn.net/cover/5787/arxiv-1904-10318v1-nucl-th-20-apr-2019-ucinltheory-445787.webp)
Arxiv:1904.10318V1 [Nucl-Th] 20 Apr 2019 Ucinltheory
Nuclear structure investigation of even-even Sn isotopes within the covariant density functional theory Y. EL BASSEM1, M. OULNE2 High Energy Physics and Astrophysics Laboratory, Department of Physics, Faculty of Sciences SEMLALIA, Cadi Ayyad University, P.O.B. 2390, Marrakesh, Morocco. Abstract The current investigation aims to study the ground-state properties of one of the most interesting isotopic chains in the periodic table, 94−168Sn, from the proton drip line to the neutron drip line by using the covariant density functional theory, which is a modern theoretical tool for the description of nuclear structure phenomena. The physical observables of interest include the binding energy, separation energy, two-neutron shell gap, rms-radii for protons and neutrons, pairing energy and quadrupole deformation. The calculations are performed for a wide range of neutron numbers, starting from the proton-rich side up to the neutron-rich one, by using the density- dependent meson-exchange and the density dependent point-coupling effec- tive interactions. The obtained results are discussed and compared with available experimental data and with the already existing results of rela- tivistic Mean Field (RMF) model with NL3 functional. The shape phase transition for Sn isotopic chain is also investigated. A reasonable agreement is found between our calculated results and the available experimental data. Keywords: Ground-state properties, Sn isotopes, covariant density functional theory. arXiv:1904.10318v1 [nucl-th] 20 Apr 2019 1. INTRODUCTION In nuclear -

Range of Usefulness of Bethe's Semiempirical Nuclear Mass Formula
RANGE OF ..USEFULNESS OF BETHE' S SEMIEMPIRIC~L NUCLEAR MASS FORMULA by SEKYU OBH A THESIS submitted to OREGON STATE COLLEGE in partial fulfillment or the requirements tor the degree of MASTER OF SCIENCE June 1956 TTPBOTTDI Redacted for Privacy lrrt rtllrt ?rsfirror of finrrtor Ia Ohrr;r ef lrJer Redacted for Privacy Redacted for Privacy 0hrtrurn of tohoot Om0qat OEt ttm Redacted for Privacy Dru of 0rrdnrtr Sohdbl Drta thrrlr tr prrrEtrl %.ifh , 1,,r, ," r*(,-. ttpo{ by Brtty Drvlr ACKNOWLEDGMENT The author wishes to express his sincere appreciation to Dr. G. L. Trigg for his assistance and encouragement, without which this study would not have been concluded. The author also wishes to thank Dr. E. A. Yunker for making facilities available for these calculations• • TABLE OF CONTENTS Page INTRODUCTION 1 NUCLEAR BINDING ENERGIES AND 5 SEMIEMPIRICAL MASS FORMULA RESEARCH PROCEDURE 11 RESULTS 17 CONCLUSION 21 DATA 29 f BIBLIOGRAPHY 37 RANGE OF USEFULNESS OF BETHE'S SEMIEMPIRICAL NUCLEAR MASS FORMULA INTRODUCTION The complicated experimental results on atomic nuclei haYe been defying definite interpretation of the structure of atomic nuclei for a long tfme. Even though Yarious theoretical methods have been suggested, based upon the particular aspects of experimental results, it has been impossible to find a successful theory which suffices to explain the whole observed properties of atomic nuclei. In 1936, Bohr (J, P• 344) proposed the liquid drop model of atomic nuclei to explain the resonance capture process or nuclear reactions. The experimental evidences which support the liquid drop model are as follows: 1. Substantially constant density of nuclei with radius R - R Al/3 - 0 (1) where A is the mass number of the nucleus and R is the constant of proportionality 0 with the value of (1.5! 0.1) x 10-lJcm~ 2. -
Modern Physics to Which He Did This Observation, Usually to Within About 0.1%
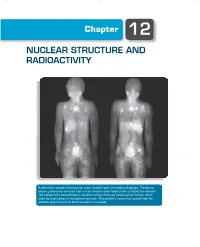
Chapter 12 NUCLEAR STRUCTURE AND RADIOACTIVITY Radioactive isotopes have proven to be valuable tools for medical diagnosis. The photo shows gamma-ray emission from a man who has been treated with a radioactive element. The radioactivity concentrates in locations where there are active cancer tumors, which show as bright areas in the gamma-ray scan. This patient’s cancer has spread from his prostate gland to several other locations in his body. 370 Chapter 12 | Nuclear Structure and Radioactivity The nucleus lies at the center of the atom, occupying only 10−15 of its volume but providing the electrical force that holds the atom together. Within the nucleus there are Z positive charges. To keep these charges from flying apart, the nuclear force must supply an attraction that overcomes their electrical repulsion. This nuclear force is the strongest of the known forces; it provides nuclear binding energies that are millions of times stronger than atomic binding energies. There are many similarities between atomic structure and nuclear structure, which will make our study of the properties of the nucleus somewhat easier. Nuclei are subject to the laws of quantum physics. They have ground and excited states and emit photons in transitions between the excited states. Just like atomic states, nuclear states can be labeled by their angular momentum. There are, however, two major differences between the study of atomic and nuclear properties. In atomic physics, the electrons experience the force provided by an external agent, the nucleus; in nuclear physics, there is no such external agent. In contrast to atomic physics, in which we can often consider the interactions among the electrons as a perturbation to the primary interaction between electrons and nucleus, in nuclear physics the mutual interaction of the nuclear constituents is just what provides the nuclear force, so we cannot treat this complicated many- body problem as a correction to a single-body problem. -

R-Process Nucleosynthesis: on the Astrophysical Conditions
r-process nucleosynthesis: on the astrophysical conditions and the impact of nuclear physics input r-Prozess Nukleosynthese: Über die astrophysikalischen Bedingungen und den Einfluss kernphysikalischer Modelle Zur Erlangung des Grades eines Doktors der Naturwissenschaften (Dr. rer. nat.) genehmigte Dissertation von M.Sc. Dirk Martin, geb. in Rüsselsheim Tag der Einreichung: 07.02.2017, Tag der Prüfung: 29.05.2017 1. Gutachten: Prof. Dr. Almudena Arcones Segovia 2. Gutachten: Prof. Dr. Jochen Wambach Fachbereich Physik Institut für Kernphysik Theoretische Astrophysik r-process nucleosynthesis: on the astrophysical conditions and the impact of nuclear physics input r-Prozess Nukleosynthese: Über die astrophysikalischen Bedingungen und den Einfluss kernphysikalis- cher Modelle Genehmigte Dissertation von M.Sc. Dirk Martin, geb. in Rüsselsheim 1. Gutachten: Prof. Dr. Almudena Arcones Segovia 2. Gutachten: Prof. Dr. Jochen Wambach Tag der Einreichung: 07.02.2017 Tag der Prüfung: 29.05.2017 Darmstadt 2017 — D 17 Bitte zitieren Sie dieses Dokument als: URN: urn:nbn:de:tuda-tuprints-63017 URL: http://tuprints.ulb.tu-darmstadt.de/6301 Dieses Dokument wird bereitgestellt von tuprints, E-Publishing-Service der TU Darmstadt http://tuprints.ulb.tu-darmstadt.de [email protected] Die Veröffentlichung steht unter folgender Creative Commons Lizenz: Namensnennung – Keine kommerzielle Nutzung – Keine Bearbeitung 4.0 International https://creativecommons.org/licenses/by-nc-nd/4.0/ Für meine Uroma Helene. Abstract The origin of the heaviest elements in our Universe is an unresolved mystery. We know that half of the elements heavier than iron are created by the rapid neutron capture process (r-process). The r-process requires an ex- tremely neutron-rich environment as well as an explosive scenario. -

Final Excitation Energy of Fission Fragments
Final excitation energy of fission fragments Karl-Heinz Schmidt and Beatriz Jurado CENBG, CNRS/IN2P3, Chemin du Solarium, B. P. 120, 33175 Gradignan, France Abstract: We study how the excitation energy of the fully accelerated fission fragments is built up. It is stressed that only the intrinsic excitation energy available before scission can be exchanged between the fission fragments to achieve thermal equilibrium. This is in contradiction with most models used to calculate prompt neutron emission where it is assumed that the total excitation energy of the final fragments is shared between the fragments by the condition of equal temperatures. We also study the intrinsic excitation- energy partition according to a level density description with a transition from a constant- temperature regime to a Fermi-gas regime. Complete or partial excitation-energy sorting is found at energies well above the transition energy. PACS: 24.75.+i, 24.60.Dr, 21.10.Ma Introduction: The final excitation energy found in the fission fragments, that is, the excitation energy of the fully accelerated fission fragments, and in particular its variation with the fragment mass, provides fundamental information on the fission process as it is influenced by the dynamical evolution of the fissioning system from saddle to scission and by the scission configuration, namely the deformation of the nascent fragments. The final fission-fragment excitation energy determines the number of prompt neutrons and gamma rays emitted. Therefore, this quantity is also of great importance for applications in nuclear technology. To properly calculate the value of the final excitation energy and its partition between the fragments one has to understand the mechanisms that lead to it. -

Semi-Empirical Mass Formula 3.3 4.6 7.2 Ch 4
Lecture 3 Krane Enge Cohen Willaims NUCLEAR PROPERTIES 1 Binding energy and stability Semi-empirical mass formula 3.3 4.6 7.2 Ch 4 2 Nuclear Spin 3.4 1.5 1.6 8.6 3 Magnetic dipole moment 3.5 1.7 1.6 8.7 4 Shape Electric Quadrupole moment 3.5 1.8 1.6 3.9, 8.8 Problems 1 From the table of nuclear masses given in the text, calculate the binding energy B, and B/A for 116Sn. 2 Calculate the various terms in the expression for the SEMF for 116Sn. From them determine B, B/A, and the mass. Compare the results with the values you obtained in question 1. 3 Using the details in the attached sheet, which is copied from Krane pages 70-72, confirm that 125Te is the most stable isobar with A=127. Also calculate the BE for both 127Te and 127I. , and thereby the energy of the β-decay. Is the β-decay β+ or β-? 4 Suppose the proton magnetic dipole moment were to be interpreted as due to the rotation of a positive uniform charge distribution of radius R, spinning about its axis with angular vel ω. a. By integrating over the charge distribution show that µ= eωR2/5 b. Using the classical relation for AM and ω, show that ωR2 = s/0.4 m2 c. Show that µ= (e/ 2m)s (analogous to Krane equ 3.32 µ= (e/ 2m)l 5. Calculate the electric quadrupole moment of a charge of magnitude Ze distributed over a ring of radius R with the axis along (a) the z-axis (b) the x-axis Tutorial Tuesday lunchtime room 210 on podium 15 Lecture 2 Review 1. -

Nuclear Reaction Rates
9 – Nuclear reaction rates introduc)on to Astrophysics, C. Bertulani, Texas A&M-Commerce 1 9.1 – Nuclear decays: Neutron decay By increasing the neutron number, the gain in binding energy of a nucleus due to the volume term is exceeded by the loss due to the growing asymmetry term. After that, no more neutrons can be bound, the neutron drip line is reached beyond which, neutron decay occurs: (Z, N) à (Z, N - 1) + n (9.1) Q-value: Qn = m(Z, N) - m(Z, N-1) - mn (9.2) Neutron Separation Energy Sn Sn(Z, N) = m(Z, N-1) + mn - m(Z, N) = -Qn for n-decay (9.3) Neutron drip line: Sn = 0 Beyond the drip line Sn < 0 neutron unbound Gamma-decay • When a reaction places a nucleus in an excited state, it drops to the lowest energy through gamma emission • Excited states and decays in nuclei are similar to atoms: (a) they conserve angular momentum and parity, (b) the photon has spin = 1 and parity = -1 • For orbital with angular momentum L, parity is given by P= (-1)L • To first order, the photon carries away the energy from an electric dipole transition in nuclei. But in nuclei it is easier to have higher order terms in than in atoms. introduc)on to Astrophysics, C. Bertulani, Texas A&M-Commerce 2 Proton decay Proton Separation Energy Sp: Sp(Z,N) = m(Z-1,N) + mp - m(Z,N) (9.4) Proton drip line: Sp = 0 beyond the drip line: Sp < 0 the nuclei are proton unbound α-decay α-decay is the emission of an a particle ( = 4He nucleus) The Q-value for a decay is given by Q "m(Z,A) m(Z 2,A 4) m $c2 α = # − − − − α % (9.5) Decay lifetimes Nuclear lifetimes for decay through all the modes described so far depend not only on the Q-values, but also on the conservation of other physical quantities, such as angular momentum. -

Handbook for Calculations of Nuclear Reaction Data, RIPL-2 Reference Input Parameter Library-2
IAEA-TECDOC-1506 Handbook for calculations of nuclear reaction data, RIPL-2 Reference Input Parameter Library-2 Final report of a coordinated research project August 2006 IAEA-TECDOC-1506 Handbook for calculations of nuclear reaction data, RIPL-2 Reference Input Parameter Library-2 Final report of a coordinated research project August 2006 The originating Section of this publication in the IAEA was: Nuclear Data Section International Atomic Energy Agency Wagramer Strasse 5 P.O. Box 100 A-1400 Vienna, Austria HANDBOOK FOR CALCULATIONS OF NUCLEAR REACTION DATA, RIPL-2 IAEA, VIENNA, 2006 IAEA-TECDOC-1506 ISBN 92–0–105206–5 ISSN 1011–4289 © IAEA, 2006 Printed by the IAEA in Austria August 2006 FOREWORD Nuclear data for applications constitute an integral part of the IAEA programme of ac- tivities. When considering low-energy nuclear reactions induced with light particles, such as neutrons, protons, deuterons, alphas and photons, a broad range of applications are addressed, from nuclear power reactors and shielding design through cyclotron production of medical radioisotopes and radiotherapy to transmutation of nuclear waste. All these and many other applications require a detailed knowledge of production cross sections, spectra of emitted particles and their angular distributions. A long-standing problem of how to meet the nuclear data needs of the future with limited experimental resources puts a considerable demand upon nuclear model compu- tation capabilities. Originally, almost all nuclear data were provided by measurement programmes. Over time, theoretical understanding of nuclear phenomena has reached a high degree of reliability, and nuclear modeling has become standard practice in nuclear data evaluations (with measurements remaining critical for data testing and benchmark- ing). -

Nuclear Physics and Astrophysics SPA5302, 2019 Chris Clarkson, School of Physics & Astronomy [email protected]
Nuclear Physics and Astrophysics SPA5302, 2019 Chris Clarkson, School of Physics & Astronomy [email protected] These notes are evolving, so please let me know of any typos, factual errors etc. They will be updated weekly on QM+ (and may include updates to early parts we have already covered). Note that material in purple ‘Digression’ boxes is not examinable. Updated 16:29, on 05/12/2019. Contents 1 Basic Nuclear Properties4 1.1 Length Scales, Units and Dimensions............................7 2 Nuclear Properties and Models8 2.1 Nuclear Radius and Distribution of Nucleons.......................8 2.1.1 Scattering Cross Section............................... 12 2.1.2 Matter Distribution................................. 18 2.2 Nuclear Binding Energy................................... 20 2.3 The Nuclear Force....................................... 24 2.4 The Liquid Drop Model and the Semi-Empirical Mass Formula............ 26 2.5 The Shell Model........................................ 33 2.5.1 Nuclei Configurations................................ 44 3 Radioactive Decay and Nuclear Instability 48 3.1 Radioactive Decay...................................... 49 CONTENTS CONTENTS 3.2 a Decay............................................. 56 3.2.1 Decay Mechanism and a calculation of t1/2(Q) .................. 58 3.3 b-Decay............................................. 62 3.3.1 The Valley of Stability................................ 64 3.3.2 Neutrinos, Leptons and Weak Force........................ 68 3.4 g-Decay........................................... -

Magic Numbers Nucleon Separation Energies
Magic numbers Experimental evidence for nuclear shell structure: + proton & neutron separation energies show similar behaviour to atomic ionization energies ( Fig. 5.1 & 5.2) Smooth increases correspond to filling of shell; sudden jumps indicate transitions to next shell. + Neutron capture probability drops drastically for nuclei with N =..., 50, 82, 126 ( Fig. 5.3b) Magic numbers of nucleons: nuclei with Z or N = 2, 8, 20, 28, 50, 82, 126 are extremely stable. Nuclei with Z and N magic numbers are said to be doubly magic e.g., 78Ni. P. Teixeira-Dias PH2510 - Atomic and Nuclear Physics Royal Holloway Univ of London Nucleon separation energies Compare ionization energies in atoms... ...with n/p separation energies in nucleii: c KS Krane Figure 5.1 c KS Krane Figure 5.2 P. Teixeira-Dias PH2510 - Atomic and Nuclear Physics Royal Holloway Univ of London The nuclear shell model (I) Neutron capture probability + Try to account for the observed magic numbers: . The harmonic oscillator potential and the infinite square well potential manage to reproduce some of these magic numbers... but not all: c KS Krane Figure 5.3 Figure 1: Shell strucyure obtained with infinite well and harmonic oscillator potentials. The capacity of each level is + marked drop in neutron capture probability for indicated to its right. Large gaps occur between levels, which we associate with closed shells. The circled numbers indicate the total nuclei with N = 28, 50, 82, 126 number of nucleons at each shell closure. c KS Krane, Figure 5.4 P. Teixeira-Dias PH2510 - Atomic and Nuclear Physics Royal Holloway Univ of London P. -

Nuclear Binding Energy 1 Nuclear Binding Energy
Nuclear binding energy 1 Nuclear binding energy Nuclear binding energy is the energy required to split a nucleus of an atom into its component parts. The component parts are neutrons and protons, which are collectively called nucleons. The binding energy of nuclei is always a positive number, since all nuclei require net energy to separate them into individual protons and neutrons. Thus, the mass of an atom's nucleus is always less than the sum of the individual masses of the constituent protons and neutrons when separated. This notable difference is a measure of the nuclear binding energy, which is a result of forces that hold the nucleus together. Because these forces result in the removal of energy when the nucleus is formed, and this energy has mass, mass is removed from the total mass of the original particles, and the mass is missing in the resulting nucleus. This missing mass is known as the mass defect, and represents the energy released when the nucleus is formed. Nuclear binding energy can also apply to situations when the nucleus splits into fragments composed of more than one nucleon, and in this case the binding energies for the fragments (as compared to the whole) may be either positive or negative, depending on where the parent nucleus and the daughter fragments fall on the nuclear binding energy curve (see below). If new binding energy is available when light nuclei fuse, or when heavy nuclei split, either of these processes result releases the binding energy. This energy—available as nuclear energy—can be used to produce nuclear power or build nuclear weapons. -

Nuclear and Nuclear Excitation Energies
ANTIBES (France ) 20 - 25 JUNE, 1994 NUCLEAR AND NUCLEAR EXCITATION ENERGIES ' ~ 9£»г* Sponsored by : CNRS/IN2P3 cl Laboratoires CEA/DAM - CEA/DSM IUR1SYS MESURES Résumés des contributions Abstracts of contributed papers ;.%•• I tpîi^ I •3' ï^^rrfV-- ^^?sji;>4.ô <,!!•*• ANTIBES, France June 20-25,1994 NUCLEAR SHAPES AND NUCLEAR STRUCTURE AT LOW EXCITATION ENERGIES Abstracts of contributed papers Résumés des contributions Edited by F. DYKSTRA, D. GOUTTE, J. SAUVAGE and M. VERGNES Institut de Physique Nucléaire F-91406 ORSAY Cedex France This volume has been prepared by Miss C. Vogelaer Ce fascicule a été préparé par Mette C. Vogelaer International Committee Comité International M. ARNOULD (Bruxelles) J. AYSTO (Jyvâskylà) J. BAUCHE (Orsay) D. BES (Buenos Aires) P. BONCHE (Saclay) H. BORNER (Grenoble) R.F. CASTEN (Brookhaven) H. DOUBRE (Orsay) D. HABS (Heidelberg) I. HAMAMOTO (Lwid; K. HEYDE (Gent) R.V.F. JANSSENS (Argonne) B. JONSON (Gôteborg) W. NAZAREWICZ (ORNL/Warsaw) E.W. OTTEN (Mamzj P. RING (Garching) H. SERGOLLE (Огаг^ P. Von BRENTANO (Ко/и) M. WEISS (Livermore) Organizing Committee Comité d'Organisation G. BARREAU (Bordeaux) J.-F. BERGER (Bruyères-le-Châtel) A. GIZON (Grenoble) D. GOUTTE (Saclay) P.H. HEENEN (Bruxelles) J. LIBERT (Orsay) M. MEYER 6Ly0rt) J. PINARD (Orsay) P. QUENTIN (Bordeaux) B. ROUSSIERE (Orjoy) J. SAUVAGE (Orsay) N. SCHULZ (Strasbourg) M. VERGNES Conférence Secrétariat Secrétariat de la Conférence F. DYKSTRA (Orsay) 232 CONTENTS - On the existence of intrinsic reflection asymmetry in Z~62, N~90 region 1 A.V. Afanasjev. S. Mizutori, I. Ragnarsson - Symmetry breaking in simple one and two level models 2 P.