Nuclear Drip Line 1 Nuclear Drip Line
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Five Common Particles
The Five Common Particles The world around you consists of only three particles: protons, neutrons, and electrons. Protons and neutrons form the nuclei of atoms, and electrons glue everything together and create chemicals and materials. Along with the photon and the neutrino, these particles are essentially the only ones that exist in our solar system, because all the other subatomic particles have half-lives of typically 10-9 second or less, and vanish almost the instant they are created by nuclear reactions in the Sun, etc. Particles interact via the four fundamental forces of nature. Some basic properties of these forces are summarized below. (Other aspects of the fundamental forces are also discussed in the Summary of Particle Physics document on this web site.) Force Range Common Particles It Affects Conserved Quantity gravity infinite neutron, proton, electron, neutrino, photon mass-energy electromagnetic infinite proton, electron, photon charge -14 strong nuclear force ≈ 10 m neutron, proton baryon number -15 weak nuclear force ≈ 10 m neutron, proton, electron, neutrino lepton number Every particle in nature has specific values of all four of the conserved quantities associated with each force. The values for the five common particles are: Particle Rest Mass1 Charge2 Baryon # Lepton # proton 938.3 MeV/c2 +1 e +1 0 neutron 939.6 MeV/c2 0 +1 0 electron 0.511 MeV/c2 -1 e 0 +1 neutrino ≈ 1 eV/c2 0 0 +1 photon 0 eV/c2 0 0 0 1) MeV = mega-electron-volt = 106 eV. It is customary in particle physics to measure the mass of a particle in terms of how much energy it would represent if it were converted via E = mc2. -

Fundamental Stellar Parameters Radiative Transfer Stellar
Fundamental Stellar Parameters Radiative Transfer Stellar Atmospheres Equations of Stellar Structure Nuclear Reactions in Stellar Interiors Binding Energy Coulomb Barrier Penetration Hydrogen Burning Reactions Burning of Helium and Heavier Elements Element Abundances Creation of Elements Heavier than Iron Introduction Stellar evolution is determined by the reactions which take place within stars: Binding energy per nucleon determines the most stable isotopes • and therefore the most probable end products of fusion and fis- sion reactions. For fusion to occur, quantum mechanical tunneling through the • repulsive Coulomb barrier must occur so that the strong nuclear force (which is a short-range force) can take over and hold the two nuclei together. Hydrogen is converted to helium by the PP-Chain and CNO- • Cycle. In due course, helium is converted to carbon and oxygen through • the 3α-reaction. Other processes, such as neutron capture reactions, produce heav- • ier elements. Binding Energy Per Nucleon { I The general description of a nuclear reaction is I(A , Z ) + J(A , Z ) K(A , Z ) + L(A , Z ) i i j j ↔ k k ` ` where A is the baryon number, nucleon number or nuclear mass of nucleus N and • n Z is the nuclear charge of nucleus N. • n The nucleus of any element (or isotope) N is uniquely defined by the two integers An and Zn. Note also that anti-particles have the opposite charge to their corresponding particle. In any nuclear reaction, the following must be conserved: the baryon number (protons, neutrons and their anti-particles), • the lepton number (electrons, positrons, neutrinos and anti-neutrinos) and • charge. -

Probing Pulse Structure at the Spallation Neutron Source Via Polarimetry Measurements
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 5-2017 Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements Connor Miller Gautam University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Nuclear Commons Recommended Citation Gautam, Connor Miller, "Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements. " Master's Thesis, University of Tennessee, 2017. https://trace.tennessee.edu/utk_gradthes/4741 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Connor Miller Gautam entitled "Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Physics. Geoffrey Greene, Major Professor We have read this thesis and recommend its acceptance: Marianne Breinig, Nadia Fomin Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements A Thesis Presented for the Master of Science Degree The University of Tennessee, Knoxville Connor Miller Gautam May 2017 c by Connor Miller Gautam, 2017 All Rights Reserved. -

Qcd in Heavy Quark Production and Decay
QCD IN HEAVY QUARK PRODUCTION AND DECAY Jim Wiss* University of Illinois Urbana, IL 61801 ABSTRACT I discuss how QCD is used to understand the physics of heavy quark production and decay dynamics. My discussion of production dynam- ics primarily concentrates on charm photoproduction data which is compared to perturbative QCD calculations which incorporate frag- mentation effects. We begin our discussion of heavy quark decay by reviewing data on charm and beauty lifetimes. Present data on fully leptonic and semileptonic charm decay is then reviewed. Mea- surements of the hadronic weak current form factors are compared to the nonperturbative QCD-based predictions of Lattice Gauge The- ories. We next discuss polarization phenomena present in charmed baryon decay. Heavy Quark Effective Theory predicts that the daugh- ter baryon will recoil from the charmed parent with nearly 100% left- handed polarization, which is in excellent agreement with present data. We conclude by discussing nonleptonic charm decay which are tradi- tionally analyzed in a factorization framework applicable to two-body and quasi-two-body nonleptonic decays. This discussion emphasizes the important role of final state interactions in influencing both the observed decay width of various two-body final states as well as mod- ifying the interference between Interfering resonance channels which contribute to specific multibody decays. "Supported by DOE Contract DE-FG0201ER40677. © 1996 by Jim Wiss. -251- 1 Introduction the direction of fixed-target experiments. Perhaps they serve as a sort of swan song since the future of fixed-target charm experiments in the United States is A vast amount of important data on heavy quark production and decay exists for very short. -

Redalyc.Projected Shell Model Description for Nuclear Isomers
Revista Mexicana de Física ISSN: 0035-001X [email protected] Sociedad Mexicana de Física A.C. México Sun, Yang Projected shell model description for nuclear isomers Revista Mexicana de Física, vol. 54, núm. 3, diciembre, 2008, pp. 122-128 Sociedad Mexicana de Física A.C. Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=57016055020 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative REVISTA MEXICANA DE FISICA´ S 54 (3) 122–128 DICIEMBRE 2008 Projected shell model description for nuclear isomers Yang Sun Department of Physics, Shanghai Jiao Tong University, Shanghai 200240, P.R. China, Joint Institute for Nuclear Astrophysics, University of Notre Dame, Notre Dame, Indiana 46545, USA. Recibido el 10 de marzo de 2008; aceptado el 7 de mayo de 2008 The study of nuclear isomer properties is a current research focus. To describe isomers, we present a method based on the Projected Shell Model. Two kinds of isomers, K-isomers and shape isomers, are discussed. For the K-isomer treatment, K-mixing is properly implemented in the model. It is found however that in order to describe the strong K-violation more efficiently, it may be necessary to further introduce triaxiality into the shell model basis. To treat shape isomers, a scheme is outlined which allows mixing those configurations belonging to different shapes. Keywords: Shell model; nuclear energy levels. Se estudian las propiedades de isomeros´ nucleares a traves´ del modelo de capas proyectadas. -

B2.IV Nuclear and Particle Physics
B2.IV Nuclear and Particle Physics A.J. Barr February 13, 2014 ii Contents 1 Introduction 1 2 Nuclear 3 2.1 Structure of matter and energy scales . 3 2.2 Binding Energy . 4 2.2.1 Semi-empirical mass formula . 4 2.3 Decays and reactions . 8 2.3.1 Alpha Decays . 10 2.3.2 Beta decays . 13 2.4 Nuclear Scattering . 18 2.4.1 Cross sections . 18 2.4.2 Resonances and the Breit-Wigner formula . 19 2.4.3 Nuclear scattering and form factors . 22 2.5 Key points . 24 Appendices 25 2.A Natural units . 25 2.B Tools . 26 2.B.1 Decays and the Fermi Golden Rule . 26 2.B.2 Density of states . 26 2.B.3 Fermi G.R. example . 27 2.B.4 Lifetimes and decays . 27 2.B.5 The flux factor . 28 2.B.6 Luminosity . 28 2.C Shell Model § ............................. 29 2.D Gamma decays § ............................ 29 3 Hadrons 33 3.1 Introduction . 33 3.1.1 Pions . 33 3.1.2 Baryon number conservation . 34 3.1.3 Delta baryons . 35 3.2 Linear Accelerators . 36 iii CONTENTS CONTENTS 3.3 Symmetries . 36 3.3.1 Baryons . 37 3.3.2 Mesons . 37 3.3.3 Quark flow diagrams . 38 3.3.4 Strangeness . 39 3.3.5 Pseudoscalar octet . 40 3.3.6 Baryon octet . 40 3.4 Colour . 41 3.5 Heavier quarks . 43 3.6 Charmonium . 45 3.7 Hadron decays . 47 Appendices 48 3.A Isospin § ................................ 49 3.B Discovery of the Omega § ...................... -

CHEM 1412. Chapter 21. Nuclear Chemistry (Homework) Ky
CHEM 1412. Chapter 21. Nuclear Chemistry (Homework) Ky Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 1. Consider the following statements about the nucleus. Which of these statements is false? a. The nucleus is a sizable fraction of the total volume of the atom. b. Neutrons and protons together constitute the nucleus. c. Nearly all the mass of an atom resides in the nucleus. d. The nuclei of all elements have approximately the same density. e. Electrons occupy essentially empty space around the nucleus. ____ 2. A term that is used to describe (only) different nuclear forms of the same element is: a. isotopes b. nucleons c. shells d. nuclei e. nuclides ____ 3. Which statement concerning stable nuclides and/or the "magic numbers" (such as 2, 8, 20, 28, 50, 82 or 128) is false? a. Nuclides with their number of neutrons equal to a "magic number" are especially stable. b. The existence of "magic numbers" suggests an energy level (shell) model for the nucleus. c. Nuclides with the sum of the numbers of their protons and neutrons equal to a "magic number" are especially stable. d. Above atomic number 20, the most stable nuclides have more protons than neutrons. e. Nuclides with their number of protons equal to a "magic number" are especially stable. ____ 4. The difference between the sum of the masses of the electrons, protons and neutrons of an atom (calculated mass) and the actual measured mass of the atom is called the ____. a. isotopic mass b. -

Nuclear Models: Shell Model
LectureLecture 33 NuclearNuclear models:models: ShellShell modelmodel WS2012/13 : ‚Introduction to Nuclear and Particle Physics ‘, Part I 1 NuclearNuclear modelsmodels Nuclear models Models with strong interaction between Models of non-interacting the nucleons nucleons Liquid drop model Fermi gas model ααα-particle model Optical model Shell model … … Nucleons interact with the nearest Nucleons move freely inside the nucleus: neighbors and practically don‘t move: mean free path λ ~ R A nuclear radius mean free path λ << R A nuclear radius 2 III.III. ShellShell modelmodel 3 ShellShell modelmodel Magic numbers: Nuclides with certain proton and/or neutron numbers are found to be exceptionally stable. These so-called magic numbers are 2, 8, 20, 28, 50, 82, 126 — The doubly magic nuclei: — Nuclei with magic proton or neutron number have an unusually large number of stable or long lived nuclides . — A nucleus with a magic neutron (proton) number requires a lot of energy to separate a neutron (proton) from it. — A nucleus with one more neutron (proton) than a magic number is very easy to separate. — The first exitation level is very high : a lot of energy is needed to excite such nuclei — The doubly magic nuclei have a spherical form Nucleons are arranged into complete shells within the atomic nucleus 4 ExcitationExcitation energyenergy forfor magicm nuclei 5 NuclearNuclear potentialpotential The energy spectrum is defined by the nuclear potential solution of Schrödinger equation for a realistic potential The nuclear force is very short-ranged => the form of the potential follows the density distribution of the nucleons within the nucleus: for very light nuclei (A < 7), the nucleon distribution has Gaussian form (corresponding to a harmonic oscillator potential ) for heavier nuclei it can be parameterised by a Fermi distribution. -

Discovery of the Isotopes with Z<= 10
Discovery of the Isotopes with Z ≤ 10 M. Thoennessen∗ National Superconducting Cyclotron Laboratory and Department of Physics and Astronomy, Michigan State University, East Lansing, MI 48824, USA Abstract A total of 126 isotopes with Z ≤ 10 have been identified to date. The discovery of these isotopes which includes the observation of unbound nuclei, is discussed. For each isotope a brief summary of the first refereed publication, including the production and identification method, is presented. arXiv:1009.2737v1 [nucl-ex] 14 Sep 2010 ∗Corresponding author. Email address: [email protected] (M. Thoennessen) Preprint submitted to Atomic Data and Nuclear Data Tables May 29, 2018 Contents 1. Introduction . 2 2. Discovery of Isotopes with Z ≤ 10........................................................................ 2 2.1. Z=0 ........................................................................................... 3 2.2. Hydrogen . 5 2.3. Helium .......................................................................................... 7 2.4. Lithium ......................................................................................... 9 2.5. Beryllium . 11 2.6. Boron ........................................................................................... 13 2.7. Carbon.......................................................................................... 15 2.8. Nitrogen . 18 2.9. Oxygen.......................................................................................... 21 2.10. Fluorine . 24 2.11. Neon........................................................................................... -
Pair Energy of Proton and Neutron in Atomic Nuclei
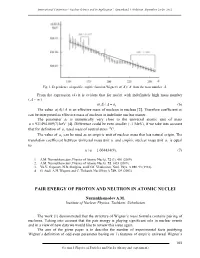
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 Fig. 1. Dependence of specific empiric function Wigner's a()/ A A from the mass number A. From the expression (4) it is evident that for nuclei with indefinitely high mass number ( A ~ ) a()/. A A a1 (6) The value a()/ A A is an effective mass of nucleon in nucleus [2]. Therefore coefficient a1 can be interpreted as effective mass of nucleon in indefinite nuclear matter. The parameter a1 is numerically very close to the universal atomic unit of mass u 931494.009(7) keV [4]. Difference could be even smaller (~1 MeV), if we take into account that for definition of u, used mass of neutral atom 12C. The value of a1 can be used as an empiric unit of nuclear mass that has natural origin. The translation coefficient between universal mass unit u and empiric nuclear mass unit a1 is equal to: u/ a1 1.004434(9). (7) 1. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72 (3), 401 (2009). 2. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72, 1435 (2009). 3. Yu.V. Gaponov, N.B. Shulgina, and D.M. Vladimirov, Nucl. Phys. A 391, 93 (1982). 4. G. Audi, A.H. Wapstra and C. Thibault, Nucl.Phys A 729, 129 (2003). PAIR ENERGY OF PROTON AND NEUTRON IN ATOMIC NUCLEI Nurmukhamedov A.M. Institute of Nuclear Physics, Tashkent, Uzbekistan The work [1] demonstrated that the structure of Wigner’s mass formula contains pairing of nucleons. Taking into account that the pair energy is playing significant role in nuclear events and in a view of new data we would like to review this issue again. -

A Measurement of the 2 Neutrino Double Beta Decay Rate of 130Te in the CUORICINO Experiment by Laura Katherine Kogler
A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment by Laura Katherine Kogler A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Physics in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Stuart J. Freedman, Chair Professor Yury G. Kolomensky Professor Eric B. Norman Fall 2011 A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment Copyright 2011 by Laura Katherine Kogler 1 Abstract A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment by Laura Katherine Kogler Doctor of Philosophy in Physics University of California, Berkeley Professor Stuart J. Freedman, Chair CUORICINO was a cryogenic bolometer experiment designed to search for neutrinoless double beta decay and other rare processes, including double beta decay with two neutrinos (2νββ). The experiment was located at Laboratori Nazionali del Gran Sasso and ran for a period of about 5 years, from 2003 to 2008. The detector consisted of an array of 62 TeO2 crystals arranged in a tower and operated at a temperature of ∼10 mK. Events depositing energy in the detectors, such as radioactive decays or impinging particles, produced thermal pulses in the crystals which were read out using sensitive thermistors. The experiment included 4 enriched crystals, 2 enriched with 130Te and 2 with 128Te, in order to aid in the measurement of the 2νββ rate. The enriched crystals contained a total of ∼350 g 130Te. The 128-enriched (130-depleted) crystals were used as background monitors, so that the shared backgrounds could be subtracted from the energy spectrum of the 130- enriched crystals. -

Photofission Cross Sections of 238U and 235U from 5.0 Mev to 8.0 Mev Robert Andrew Anderl Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1972 Photofission cross sections of 238U and 235U from 5.0 MeV to 8.0 MeV Robert Andrew Anderl Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Nuclear Commons, and the Oil, Gas, and Energy Commons Recommended Citation Anderl, Robert Andrew, "Photofission cross sections of 238U and 235U from 5.0 MeV to 8.0 MeV " (1972). Retrospective Theses and Dissertations. 4715. https://lib.dr.iastate.edu/rtd/4715 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This dissertation was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction, 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity, 2.