Research Dissertation Title: the Pattern of Hearing Loss As Seen at the University of Benin Teaching Hospital, Benin City
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BMC Ear, Nose and Throat Disorders Biomed Central
BMC Ear, Nose and Throat Disorders BioMed Central Case report Open Access Acute unilateral hearing loss as an unusual presentation of cholesteatoma Daniel Thio*1, Shahzada K Ahmed2 and Richard C Bickerton3 Address: 1Department of Otorhinolaryngology, South Warwickshire General Hospitals NHS Trust Warwick CV34 5BW UK, 2Department of Otorhinolaryngology, South Warwickshire General Hospitals NHS Trust Warwick CV34 5BW UK and 3Department of Otorhinolaryngology, South Warwickshire General Hospitals NHS Trust Warwick CV34 5BW UK Email: Daniel Thio* - [email protected]; Shahzada K Ahmed - [email protected]; Richard C Bickerton - [email protected] * Corresponding author Published: 18 September 2005 Received: 10 July 2005 Accepted: 18 September 2005 BMC Ear, Nose and Throat Disorders 2005, 5:9 doi:10.1186/1472-6815-5-9 This article is available from: http://www.biomedcentral.com/1472-6815/5/9 © 2005 Thio et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Cholesteatomas are epithelial cysts that contain desquamated keratin. Patients commonly present with progressive hearing loss and a chronically discharging ear. We report an unusual presentation of the disease with an acute hearing loss suffered immediately after prolonged use of a pneumatic drill. Case presentation: A 41 year old man with no previous history of ear problems presented with a sudden loss of hearing in his right ear immediately following the prolonged use of a pneumatic drill on concrete. -

Bedside Neuro-Otological Examination and Interpretation of Commonly
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2004.054478 on 24 November 2004. Downloaded from BEDSIDE NEURO-OTOLOGICAL EXAMINATION AND INTERPRETATION iv32 OF COMMONLY USED INVESTIGATIONS RDavies J Neurol Neurosurg Psychiatry 2004;75(Suppl IV):iv32–iv44. doi: 10.1136/jnnp.2004.054478 he assessment of the patient with a neuro-otological problem is not a complex task if approached in a logical manner. It is best addressed by taking a comprehensive history, by a Tphysical examination that is directed towards detecting abnormalities of eye movements and abnormalities of gait, and also towards identifying any associated otological or neurological problems. This examination needs to be mindful of the factors that can compromise the value of the signs elicited, and the range of investigative techniques available. The majority of patients that present with neuro-otological symptoms do not have a space occupying lesion and the over reliance on imaging techniques is likely to miss more common conditions, such as benign paroxysmal positional vertigo (BPPV), or the failure to compensate following an acute unilateral labyrinthine event. The role of the neuro-otologist is to identify the site of the lesion, gather information that may lead to an aetiological diagnosis, and from there, to formulate a management plan. c BACKGROUND Balance is maintained through the integration at the brainstem level of information from the vestibular end organs, and the visual and proprioceptive sensory modalities. This processing takes place in the vestibular nuclei, with modulating influences from higher centres including the cerebellum, the extrapyramidal system, the cerebral cortex, and the contiguous reticular formation (fig 1). -

Cochlear Implants in Unilateral Hearing Loss for Tinnitus Suppression
Central Annals of Otolaryngology and Rhinology Review Article *Corresponding author Mohamed Salah Elgandy, Department of Otolaryngology-Head and Neck Surgery, University of Cochlear Implants in Unilateral Iowa hospital and clinics, 200 Hawkins drive, Iowa, Iowa City 52242.PFP 21167, USA, Tel; +1(319)519-3862; Email; Hearing Loss for Tinnitus Submitted: 19 January 2019 Accepted: 06 February 2019 Suppression Published: 08 February 2019 ISSN: 2379-948X 1,2 2,3 Mohamed Salah Elgandy *and Richard S. Tyler Copyright 1Department of Otolaryngology-Head and Neck Surgery, Zagazig University, Egypt © 2019 Elgandy et al. 2Department of Otolaryngology-Head and Neck Surgery, University of Iowa, USA 3Department of Communication Sciences and Disorders, University of Iowa, USA OPEN ACCESS Keywords Abstract • Unilateral hearing loss Tinnitus is a pervasive symptom that can affect many people with hearing loss. • Tinnitus It is found that its incidence is increasing due to accompanying occupational and • Electrical stimulation environmental noise. Even, there is no standard treatment is present up till now, but • Cochlear implants cochlear implants (CIs) positive effects are well proven and documented. This article provides an overview of many publicly available reports about cochlear implants and tinnitus, with review of several articles demonstrating the benefit of cochlear implants for unilateral hearing loss and tinnitus. We believe that this approach will help many, and should be considered as standard practice and reimbursed. INTRODUCTION An increase in rate. Unilateral hearing loss affecting approximately18.1 million • Decrease in rate persons in the United States [1]. Patients with unilateral deafness • Periodic activity frequently also experience tinnitus, which can have a profound • Synchronous activity cross neurons has been associated with an increased incidence of depression, impact on an individual’s quality of life. -

32 3. UNADDRESSED HEARING PROBLEMS This Section Contains
3. UNADDRESSED HEARING PROBLEMS This section contains: a description of the types of hearing problems that affect children; the prevalence of hearing problems; unmet needs for hearing screening, diagnosis and treatment; and evidence on the learning consequences of unaddressed hearing problems. Definitions Normal hearing was defined in 1965 by the American Academy of Ophthalmology and Otolaryngology (AAOO) as any hearing loss up to 26 dB. This level of hearing loss is the point at which an individual begins to find it difficult to understand typical speech in a quiet environment. The AAOO guidelines around normal hearing have not changed since this cutoff was established and are supported by the American Medical Association and the American Academy of Audiology. Hearing deficits are categorized and defined in various ways, and there is variation in defining levels of hearing loss across countries, states, and health care providers. There are no widely agreed upon definitions for all types and levels of hearing loss. The American Speech-Language-Hearing Association (ASHA) categorizes and defines hearing loss primarily by type, degree, and configuration.103 Type of Hearing Loss (as defined by ASHA) ● Sensorineural hearing loss (SNHL) is the most common type of permanent hearing loss and “happens when there is damage to the inner ear (cochlea) or to the nerve pathways from the inner ear to the brain.” Audible speech may be unclear or sound muffled. This type of hearing loss can usually not be corrected with medical treatment or surgery. SNHL can be caused by: ○ Drugs that are toxic to hearing ○ Hearing loss that runs in the family (genetic or hereditary) ○ Head trauma ○ Malformation of the inner ear ○ Exposure to loud noise ● Conductive hearing loss “occurs when sound is not sent easily through the outer ear canal to the eardrum and the tiny bones (ossicles) of the middle ear.” Sounds will seem softer and less easy to hear. -

A Unilateral Cochlear Implant for Tinnitus
REVIEW PAPER DOI: 10.5935/0946-5448.20180022 International Tinnitus Journal. 2018;22(2):128-132. A Unilateral Cochlear Implant for Tinnitus Mohamed Salah Elgandy1 Richard Tyler2,3 Camille Dunn2 Marlan Hansen2 Bruce Gantz2 Abstract In recent years a growing number of Patients with unilateral hearing loss have been undergoing cochlear implantation. We provide an overview of the efficacy of cochlear implants (CIs) to rehabilitate patients with unilateral deafness with regards to sound localization, speech recognition, and tinnitus. Although CI is not yet an FDA-approved treatment for unilateral deafness, several recent studies show improvements in speech understanding, sound localization, and tinnitus. Based on encouraging results and the unique ability to restore binaural sound processing, the benefits to many as an aid to their tinnitus, we argue that CIs should be offered as a treatment for unilateral deafness. Keywords: hearing loss, tinnitus, electrical stimulation, cochlear implants. 1Department of Otolaryngology-Head and Neck Surgery, Zagazig University, Egypt 2Department of Otolaryngology-Head and Neck Surgery, University of Iowa, Iowa City, USA 3Department of Communication Sciences and Disorders, University of Iowa, Iowa City, USA Send correspondence to: Mohamed Salah Elgandy Department of Otolaryngology-Head and Neck Surgery, Zagazig University, Egypt. E-mail: [email protected] Paper submitted to the ITJ-EM (Editorial Manager System) on August 30, 2018; and accepted on September 10, 2018. International Tinnitus Journal, Vol. 22, No 2 (2018) 128 www.tinnitusjournal.com INTRODUCTION disturbances. It is important to note that the therapy in these situations is for depression and anxiety, not tinnitus. Unilateral hearing loss implies a profound sensori- neural hearing loss in one ear and no greater than a mild As with any bothersome, common disorder that lacks hearing loss in the opposite ear. -

A Giant Cholesteatoma of the Mastoid Extending Into the Foramen
Neurology International 2018; volume 10:7625 A giant cholesteatoma of the mastoid extending into Introduction Correspondence: Yuekang Zhang, Department of Neurosurgery, Post Graduate Training the foramen magnum: Cholesteatomas are benign, slowly pro- Centre, West China Hospital, Sichuan gressive lesions that are usually found in the University; 37 Guo Xue Xiang Road, A case report and review 1-5 temporal bone. They are epidermoid Chengdu, Sichuan 610041, P.R. China. of literature cysts of embryologic origin that result in Tel.: +86.18980601975 - Fax: +86.02885422490. progressive desquamation and trapping of E-mail: [email protected] Seidu A. Richard,1-3 Li Qiang,1 squamous epithelium behind an intact tym- panic membrane.6 They can be congenital Key words: Cholesteatoma, Case report, Zhi Gang Lan,1 Yuekang Zhang,1 or acquired.1,4,5 They can be classified into Dizziness, Epidermoid Cyst, Hearing loss, Chao You1 supralabyrinthine, infralabyrinthine, mas- Tinnitus. 1Department of Neurosurgery, West sive labyrinthine, infralabyrinthine-apical, Contributions: SAR conceived the project and and apical.1,4 They have the penchant for China Hospital, Sichuan University, designed the study; SAR, LQ and ZQL col- 2 China; Department of Immunology, the petrous apex, skull base, and internal lected patient’s data; YZ and CY provided Jiangsu University, China; 3Department auditory canal. Also, these lesions may technical assistance in the study; SAR pre- of Surgery, Volta Regional Hospital, Ho, infiltrate other vital soft tissue structures pared the illustration, analyzed data and wrote such as the sigmoid sinus, jugular vein and Ghana the paper. artery, and the cerebellopontine angle.1,4,5 Symptoms associated with cholesteatomas Conflict of interest: the authors declare no includes vertigo, tinnitus, otorrhea, and potential conflict of interest. -

Hearing Loss and Auditory Disorders: Outside the Clinic
Hollea Ryan, Au.D., Ph.D., CCC-A Audiology Program Director, Samford University Bethany Wenger, Au.D., CCC-A Pediatric Audiologist, Vanderbilt University Disclaimers • Hollea Ryan is employed by Samford University and receives financial compensation for her work. No conflict of interest exists for this presentation. • Bethany Wenger is employed by Vanderbilt University Medical Center and receives financial compensation for her work. She has previously worked as a consultant for a hearing protection device company and was financially compensated. No conflict of interest exists for this presentation. Hearing Loss and Auditory Disorders:Hearing Loss Outside and Auditorythe Clinic Disorders: Outside the Clinic AGENDA • Unilateral Hearing Loss • Minimal (Bilateral) Hearing Loss • Auditory Disorders • Non-clinical Settings • Noise-Induced Hearing Loss Learning Objectives At the completion of this presentation, the participant will be able to: 1) Detail current research findings regarding children with minimal hearing loss and/or noise-induced hearing loss. 2) Identify non-academic settings in which children with hearing loss struggle. 3) Summarize various treatment options for children with hearing loss that improve communication, academic performance, and/or quality of life. What the Literature is Indicating about Minimal/Unilateral Hearing Loss Hearing Care Practices Before and After UNHS (Fitzpatrick, Whittingham, & Durieux-Smith, 2013; Fitzpatrick, Durieux-Smith, & Whittingham, 2010) • Retrospectively evaluated 20 years of history related -
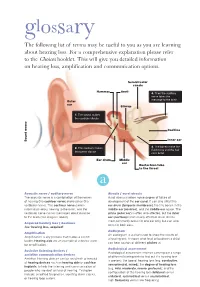
Glossary the Following List of Terms May Be Useful to You As You Are Learning About Hearing Loss
glossary The following list of terms may be useful to you as you are learning about hearing loss. For a comprehensive explanation please refer to the Choices booklet. This will give you detailed information on hearing loss, amplification and communication options. Semicircular canals Hammer Anvil 4. Then the auditory nerve takes the message to the brain. Outer ear Stirrup 1. The sound makes the eardrum vibrate Cochlea Inner ear Sound waves . The bones make the 2. The eardrum makes 3 fluid move and the hair the bones vibrate cells bend. Ear drum Middle ear Eustachian tube to the throat a Acoustic nerve / auditory nerve Atresia / aural atresia The acoustic nerve is a combination of the nerves Aural atresia involves some degree of failure of of hearing (the cochlear nerve) and balance (the development of the ear canal. It can also affect the vestibular nerve). The cochlear nerve carries ear drum (tympanic membrane), the tiny bones in the information about hearing to the brain, and the middle ear (ossicles), and the middle ear space. The vestibular nerve carries messages about balance pinna (outer ear) is often also affected, but the inner to the brain (see diagram above). ear (cochlea) is not usually affected. Aural atresia most commonly occurs in one ear only, but can also Acquired hearing loss / deafness occur in both ears. See ‘hearing loss, acquired’. Audiogram Amplification An audiogram is a chart used to show the results of Amplification is any process that makes a sound a hearing test. It shows what level of loudness a child louder. Hearing aids are an example of a device used can hear sounds of different pitches at. -

Differential Diagnosis and Treatment of Hearing Loss JON E
Differential Diagnosis and Treatment of Hearing Loss JON E. ISAACSON, M.D., and NEIL M. VORA, M.D., Milton S. Hershey Medical Center, Hershey, Pennsylvania Hearing loss is a common problem that can occur at any age and makes verbal communication difficult. The ear is divided anatomically into three sections (external, middle, and inner), and pathology contributing to hearing loss may strike one or more sections. Hearing loss can be cat- egorized as conductive, sensorineural, or both. Leading causes of conductive hearing loss include cerumen impaction, otitis media, and otosclerosis. Leading causes of sensorineural hear- ing loss include inherited disorders, noise exposure, and presbycusis. An understanding of the indications for medical management, surgical treatment, and amplification can help the family physician provide more effective care for these patients. (Am Fam Physician 2003;68:1125-32. Copyright© 2003 American Academy of Family Physicians) ore than 28 million Amer- tive, the sound will be heard best in the icans have some degree of affected ear. If the loss is sensorineural, the hearing impairment. The sound will be heard best in the normal ear. differential diagnosis of The sound remains midline in patients with hearing loss can be sim- normal hearing. Mplified by considering the three major cate- The Rinne test compares air conduction gories of loss. Conductive hearing loss occurs with bone conduction. The tuning fork is when sound conduction is impeded through struck softly and placed on the mastoid bone the external ear, the middle ear, or both. Sen- (bone conduction). When the patient no sorineural hearing loss occurs when there is a longer can hear the sound, the tuning fork is problem within the cochlea or the neural placed adjacent to the ear canal (air conduc- pathway to the auditory cortex. -

Unilateral Hearing Loss: Outcomes
UNILATERAL HEARING LOSS: OUTCOMES RECRUIT- CASE ASSESSMENT AUTHOR’S REFERENCE DESIGN MENT DEFINITION SUBJECTS TOOLS RESULTS CONCLUSIONS Bess F, Case history 60 children Only selection Total tested: Comprehensive Only 23% of children with Most important Tharpe A. data with UHL* criteria used: N = 60 with medical and UHL were identified before finding was half Case history examined in 3 from mid- UHL. educational age 5 (Mean = 5.68 years). of the children data on ways: Tennessee An audiogram case histories showed problems unilaterally region. obtained by an Subsample of Approximately 50% of 60 in educational hearing- Total group of audiologist. 25 children showed some difficulty in progress. impaired children. who satisfied educational progress. children. Ear SNHL* loss of more Results indicated 35% failed at least one Hear. 1986; Close ≥45 dB* (.5, 1, stringent st a need to re 7(1):14–9. examination 2 kHz*) in criteria. grade (most failed 1 assess these of subgroup of poorer ear and grade, although half failed children’s needs. 25 children. ≤15 dB in Group of 8 higher grades). better ear. academically 13.3% were in need of Close unsuccessful some special resource examination No experience children from assistance. of 8 with the group of academically amplification. 25. All children received unsuccessful classroom seating children from Aged 6–18 preference. the group of years. 25. 20% described by teachers as having behavioral problems. Similar finding obtained on a subset of 25 children with UHL that satisfied more stringent criteria. Small group of 8 academically unsuccessful children: Half had a right ear loss–repeated 1st grade due to “immaturity” or “hyperactivity.” Data from metro-Nashville public schools: 3.5% of children in grades K–6 failed one or more grades. -

Factors Potentially Affecting the Hearing of Petroleum Industry Workers
report no. 5/05 factors potentially affecting the hearing of petroleum industry workers Prepared for CONCAWE’s Health Management Group by: P. Hoet M. Grosjean Unité de toxicologie industrielle et pathologie professionnelle Ecole de santé publique Faculté de médecine Université catholique de Louvain (Belgium) C. Somaruga School of Occupational Health University of Milan (Italy) Reproduction permitted with due acknowledgement © CONCAWE Brussels June 2005 I report no. 5/05 ABSTRACT This report aims at giving an overview of the various factors that may influence the hearing of petroleum industry workers, including the issue of ‘ototoxic’ chemical exposure. It also provides guidance for occupational physicians on factors that need to be considered as part of health management programmes. KEYWORDS hearing, petroleum industry, hearing loss, audiometry, ototoxicity, chemicals INTERNET This report is available as an Adobe pdf file on the CONCAWE website (www.concawe.org). NOTE Considerable efforts have been made to assure the accuracy and reliability of the information contained in this publication. However, neither CONCAWE nor any company participating in CONCAWE can accept liability for any loss, damage or injury whatsoever resulting from the use of this information. This report does not necessarily represent the views of any company participating in CONCAWE. II report no. 5/05 CONTENTS Page SUMMARY IV 1. INTRODUCTION 1 2. HEARING, MECHANISMS AND TYPES OF HEARING LOSS 3 2.1. PHYSIOLOGY OF HEARING: HEARING BASICS 3 2.2. MECHANISMS AND TYPES OF HEARING LOSS 4 2.2.1. Transmission or conduction hearing loss 4 2.2.2. Sensorineural hearing loss 5 2.3. EVALUATION OF HEARING LOSS 6 3. -

Cochlear and Auditory Brainstem Implants
Medical Coverage Policy Effective Date ............................................11/15/2020 Next Review Date ......................................11/15/2021 Coverage Policy Number .................................. 0190 Cochlear and Auditory Brainstem Implants Table of Contents Related Coverage Resources Overview .............................................................. 1 Hearing Aids Coverage Policy ................................................... 1 Otoplasty and External Ear Reconstruction General Background ............................................ 4 Speech Therapy Medicare Coverage Determinations .................. 18 Coding/Billing Information .................................. 18 References ........................................................ 20 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies