Rubidium Data Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![(12) United States Patent (10) Patent N0.: US 8,304,580 B2 Nanmyo Et A]](https://docslib.b-cdn.net/cover/4589/12-united-states-patent-10-patent-n0-us-8-304-580-b2-nanmyo-et-a-144589.webp)
(12) United States Patent (10) Patent N0.: US 8,304,580 B2 Nanmyo Et A]
US008304580B2 (12) United States Patent (10) Patent N0.: US 8,304,580 B2 Nanmyo et a]. (45) Date of Patent: Nov. 6, 2012 (54) METHOD FOR PRODUCING TRIS(PER FOREIGN PATENT DOCUMENTS FLUORO-ALKANESULFONYL)METHIDE EP 0813521 B1 * 9/2000 ACID SALT JP 2000-226392 A 8/2000 JP 2000-256348 A 9/2000 (75) Inventors: Tsutomu Nanmyo, Ube (JP); Shintaro JP 2000-256348 A * 9/2000 Sasaki, Ube (JP); Takashi Kume, OTHER PUBLICATIONS KaWagoe (JP) English translation of JP-2000-256348-A; “machine translation” (73) Assignee: Central Glass Company, Limited, from JPO link at: http://WWW4.ipd1.inpit.go.jp/Tokujitu/ Ube-shi (JP) tj sogodbenkipdl accessed Oct. 25, 201 1; relevant part of document.* European Search Report dated Jun. 8, 2011 (four (4) pages). ( * ) Notice: Subject to any disclaimer, the term of this Waller et al., “Tris (tri?uoromethanesulfonyl) methide (“Tri?ide”) patent is extended or adjusted under 35 Anion: Convenient Preparation, X-ray Crystal Structures, and U.S.C. 154(b) by 528 days. Exceptional Catalytic Activity as a Counterion With Ytterbium (III) and Scandium (111)”, Journal of Organic Chemistry, vol. 64, 1999, pp. (21) Appl. N0.: 12/520,17s 2910-2913, XP-002636992. Turowsky et al., “Tris ((tri?uoromethyl) sulfonyl) methane, HC (22) PCT Filed: Dec. 18, 2007 (SO2CF3)3”, Journal of Inorganic Chemistry, vol. 27, 1988, pp. 2135-2137, XP-002636993. (86) PCT No.: PCT/JP2007/074296 International Search Report and PCT/ISN237 W/translation dated Feb. 12, 2008 (Seven (7) pages). § 371 (0X1)’ LutZ Turowsky et al., “Tris((tri?uoromethyl)sulfonyl)methane, (2), (4) Date: Jun. -

WO 2015/084416 Al 11 June 2015 (11.06.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/084416 Al 11 June 2015 (11.06.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C09K 8/05 (2006.01) C07C 51/42 (2006.01) kind of national protection available): AE, AG, AL, AM, COW 17/00 (2006.01) E21B 21/06 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US20 13/076445 HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 19 December 2013 (19. 12.2013) MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (25) Filing Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (26) Publication Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/910,976 3 December 2013 (03. 12.2013) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant: CABOT CORPORATION [US/US]; Two GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, Seaport Lane, Suite 1300, Boston, MA 01220 (US). -

Thermal Studies on Rubidium Dinitramide
University of Huddersfield Repository Charsley, Edward L., Laye, Peter G., Markham, H.M., Rooney, James J., Berger, B., Griffiths, Trevor T. and Wasko, M.P. Thermal Studies on Rubidium Dinitramide Original Citation Charsley, Edward L., Laye, Peter G., Markham, H.M., Rooney, James J., Berger, B., Griffiths, Trevor T. and Wasko, M.P. (2008) Thermal Studies on Rubidium Dinitramide. In: 35th International Pyrotechnics Seminar, 13-18 July 2008, Fort Collins, USA. This version is available at http://eprints.hud.ac.uk/id/eprint/15577/ The University Repository is a digital collection of the research output of the University, available on Open Access. Copyright and Moral Rights for the items on this site are retained by the individual author and/or other copyright owners. Users may access full items free of charge; copies of full text items generally can be reproduced, displayed or performed and given to third parties in any format or medium for personal research or study, educational or not-for-profit purposes without prior permission or charge, provided: • The authors, title and full bibliographic details is credited in any copy; • A hyperlink and/or URL is included for the original metadata page; and • The content is not changed in any way. For more information, including our policy and submission procedure, please contact the Repository Team at: [email protected]. http://eprints.hud.ac.uk/ Proceedings 35 th International Pyrotechnic Seminar, Fort Collins, USA, IPSUSA, INC, 2008, 331. Thermal Studies on Rubidium Dinitramide E.L.Charsley l, P.G.Laye 1, H.M.Markham 1, J.J.Rooney 1, B.Berger 2, T.T.Griffiths 3 and M. -

United States Patent (19) 11) 4,080,491 Kobayashi Et Al
United States Patent (19) 11) 4,080,491 Kobayashi et al. 45) Mar. 21, 1978 54) PROCESS OF PRODUCING RING-OPENING 56 References Cited POLYMERIZATION PRODUCTS U.S. PATENT DOCUMENTS (75) Inventors: Yukio Kobayashi; Takashi Ueshima; 3,798,175 3/1974 Streck .................................. 526/136 Shoichi Kobayashi, all of Yokohama, 3,856,758 - 12/1974 Ueshima ............................... 526/169 Japan 3,859,265 1/1975 Hepworth ............................ 526/281 3,959,234 5/1976 Kurosawa ............................ 526/281 Assignee: Showa Denko K.K., Tokyo, Japan (73) Primary Examiner-Paul R. Michl 21 Appl. No.: 714,833 Attorney, Agent, or Firm-Fitzpatrick, Cella, Harper & 22) Filed: Aug. 16, 1976 Scinto Foreign Application Priority Data 57 ABSTRACT (30) A process of producing a ring-opening polymerization Aug. 27, 1975 Japan ................................ 50-103060 product of a norbornene derivative containing at least Mar. 26, 1976 Japan .................................. 51-32464 one polar group or aromatic group, a norbornadiene Mar. 26, 1976 Japan .................................. 51-32465 derivative containing at least one of said groups or a Apr. 5, 1976 Japan .................................. 51-37274 cycloolefin using a catalyst system prepared from an Apr. 27, 1976 Japan .................................. 51-47268 organometallic compound and the reaction product of May 25, 1976 Japan .................................. 51-59642 tungsten oxide or molybdenum oxide and a phosphorus 51 Int. C.’................................................ C08F 4/78 pentahalide or phosphorus oxytrihalide or these com (52) U.S. C. ..... o e s p s 8 v 8 526/137; 526/113; pounds and other third components. The catalyst sys 526/127; 526/136; 526/169; 526/281; 526/308 temi possesses a high polymerization activity. (58) Field of Search .................... -

Mineral Commodity Summareis 2013
U.S. Department of the Interior U.S. Geological Survey MINERAL COMMODITY SUMMARIES 2013 Abrasives Fluorspar Mercury Silver Aluminum Gallium Mica Soda Ash Antimony Garnet Molybdenum Sodium Sulfate Arsenic Gemstones Nickel Stone Asbestos Germanium Niobium Strontium Barite Gold Nitrogen Sulfur Bauxite Graphite Peat Talc Beryllium Gypsum Perlite Tantalum Bismuth Hafnium Phosphate Rock Tellurium Boron Helium Platinum Thallium Bromine Indium Potash Thorium Cadmium Iodine Pumice Tin Cement Iron and Steel Quartz Crystal Titanium Cesium Iron Ore Rare Earths Tungsten Chromium Iron Oxide Pigments Rhenium Vanadium Clays Kyanite Rubidium Vermiculite Cobalt Lead Salt Wollastonite Copper Lime Sand and Gravel Yttrium Diamond Lithium Scandium Zeolites Diatomite Magnesium Selenium Zinc Feldspar Manganese Silicon Zirconium U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia: 2013 Manuscript approved for publication January 24, 2013. For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment— visit http://www.usgs.gov or call 1–888–ASK–USGS. For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod For sale by the Superintendent of Documents, U.S. Government Printing Office Mail: Stop IDCC; Washington, DC 20402–0001 Phone: (866) 512–1800 (toll-free); (202) 512–1800 (DC area) Fax: (202) 512–2104 Internet: bookstore.gpo.gov Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted material contained within this report. -

Everything Chemistry Poster
Common Polyatomic Ions by Charge and Ion Family Functional Groups +1 Charge -1 Charge -2 Charge -3 Charge H + - 2- 3- | Ammonium NH4 Dihydrogen phosphite H2PO3 Hydrogen phosphite HPO3 Phosphite PO3 R—OH Alcohol 1° H—C—R + - 2- 3- Hydronium H3O Dihydrogen phosphate H2PO4 Hydrogen phosphate HPO4 Phosphate PO4 | - 2- 3- H Hydrogen carbonate HCO Carbonate CO Hypophosphite PO 3 3 2 - 2- 3- H Ammonia NH3 Hydrogen sulfite HSO3 Sulfite SO3 Arsenite AsO3 R | - 2- 3- | Hydrogen sulfate HSO Sulfate SO Arsenate AsO Amine 2° H—C—R 4 4 4 R—N—R - 2- | Nitrite NO2 Thiosulfate S2O3 - 2- R’ Nitrate NO3 Silicate SIO3 -4 Charge H - 2- 4- Hydroxide OH Carbide C2 Pyrophosphate P2O7 | R—O—R C - 2- Ether 3° R”—C—R Acetate CH3COO Oxalate C2O4 - 2- | Chromite CrO2 Chromate CrO4 R’ - 2- Cyanide CN Dichromate Cr2O7 H R’” - 2- O Cyanate CNO Tartrate C4H4O6 || | - 2- Aldehyde 4° R”—C—R Thiocyanate CNS Molybdate MoO R—C—H 4 | E - 2- Superoxide O2 Peroxide O2 R’ - 2- Permanganate MnO Disulfide S 4 2 O R - 2- Amide n-butyl M Hypochlorite ClO Oxalate C2O4 || | - R—C—N—R R Chlorite ClO2 - Chlorate ClO3 Strong Acids and Bases O I - Carboxylic Acid iso-butyl Perchlorate ClO4 Acids Bases || R Hypobromite BrO- Hydrochloric Acid HCl Lithium Hydroxide LiOH R—C—OH S - Bromite BrO2 Hydrobromic Acid HBr Sodium Hydroxide NaOH O - Ester || sec-butyl Bromate BrO3 Hydroiodic Acid HI Potassium Hydroxide KOH R—C—O—R R Perbromate BrO - Nitric Acid HNO Rubidium Hydroxide RbOH T 4 3 - Hypoiodite IO Sulfuric Acid H2SO4 Cesium Hydroxide CsOH O - Ketone || tert-butyl Iodite IO2 Perchloric -

Toxic Element Clearance Profile Ratio to Creatinine
Toxic Element Clearance Profile Ratio to Creatinine JANE Order Number: DOE Toxic Elements Sulfur esults in g g reatinine esults in g g reatinine le ent e eren e ange TMP e eren e le ent e eren e ange e eren e ange ange Creatinine Concentration Collection Information TMPL Tentative Ma i u Per issi le i it Tin To i ology Tungsten T e Basi S ien e o Poisons © Genova Diagnostics · A. L. Peace-Brewer, PhD, D(ABMLI), Lab Director · CLIA Lic. #34D0655571 · Medicare Lic. #34-8475 Patient: JANE DOE Page 2 Commentary <dl = Unable to determine results due to less than detectable levels of analyte. Commentary is provided to the practitioner for educational purposes, and should not be interpreted as diagnostic or treatment recommendations. Diagnosis and treatment decisions are the responsibility of the practitioner. Reference Range Information: Elemental reference ranges were developed from a healthy population under non-provoked/non-challenged conditions. Provocation with challenge substances is expected to raise the urine level of some elements to varying degrees, often into the cautionary or TMPL range. The degree of elevation is dependent upon the element level present in the individual and the binding affinities of the challenge substance. Urine creatinine concentration is below the reference range. This may be due to increased fluid intake, a low protein diet, low body weight, or low levels of physical activity. Conditions such as diuretic use, dietary deficiencies of precursor amino acids (arginine, glycine, or methionine), malnutrition, or hypothyroidism may also lower creatinine levels. Measurement of serum creatinine or a creatinine clearance test can help determine if there are changes in renal function. -

1000004316196.Pdf
(This is a sample cover image for this issue. The actual cover is not yet available at this time.) This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Fluid Phase Equilibria 336 (2012) 34–40 Contents lists available at SciVerse ScienceDirect Fluid Phase Equilibria journa l homepage: www.elsevier.com/locate/fluid Osmotic and activity coefficients for the CsF + methanol or ethanol + water ternary systems determined by potentiometric measurements ∗ ∗ Jing Tang, Lei Wang, Shu’ni Li , Quanguo Zhai, Yucheng Jiang, Mancheng Hu Key Laboratory of Macromolecular Science of Shaanxi Province, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi’an, Shaanxi 710062, PR China a r t i c l e i n f o a b s t r a c t Article history: The thermodynamic properties of CsF in the ROH (R = methyl or ethyl) + water mixtures were determined Received 18 July 2012 using potentiometric measurements at 298.15 K with the mass fraction of ROH varying from 0.00 to 0.30. -

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

3-D Nano and Micro Structures
TM Vol. 3, No. 1 3-D Nano and Micro Structures Inks for Direct-Write 3-D Assembly Colloidal Crystal Templating Small Structures Inspiring Electrospinning Big Technologies Quantum Dots: Nanoscale Synthesis and Micron-Scale Applications 2 Introduction TM Welcome to the first 2008 issue of Material Matters™, focusing on 3-dimensional (3D) micro- and nanostructures. New techniques to create materials ordered at micro- Vol. 3 No. 1 and nanoscale drive scientific and technological advances in many areas of science and engineering. For example, 3D-patterned metal oxides could enable construction of micro-fuel cells and high-capacity batteries smaller and more energy efficient compared to presently available devices. Ordered porous materials with exceedingly large surface areas are good candidates for highly efficient catalysts and sensors. Aldrich Chemical Co., Inc. New methods for periodic patterning of semiconductor materials are fundamental Sigma-Aldrich Corporation to next-generation electronics, while individual nanoscale semiconductor structures, 6000 N. Teutonia Ave. known as quantum dots (QDs), have remarkable optical properties for optoelectronics Milwaukee, WI 53209, USA and imaging applications. And biomedical engineers are using tailored nanofibers and patterned polymeric structures to develop tissue-engineering scaffolds, drug-delivery devices and microfluidic networks. To Place Orders The broad diversity of potentially relevant material types and architectures underscores Telephone 800-325-3010 (USA) the need for new approaches to make 3D micro- and nanostructures. In this issue, FAX 800-325-5052 (USA) Introduction Professor Jennifer Lewis (University of Illinois at Urbana-Champaign) describes direct- write printing of 3D periodic arrays. This work depends on formulation of suitable Customer & Technical Services inks, many of which can be prepared using particles and polyelectrolytes available Customer Inquiries 800-325-3010 from Sigma-Aldrich®. -
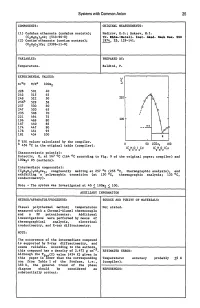
Systems with Common Anion 25
Systems with Common Anion 25 COMPONENTS: ORIGINAL MEASUREMENTS: (1) Cadmium ethanoate (cadmium acetate); Nadirov, E.G.; Bakeev, M.I. (C2H302)2Cd; [543-90-8] Tr. KhilR.-Ketall. IDst. Akad. Nauk Kaz. 8SR (2) Cesium ethanoate (cesium acetate); 1974, 25, 129-141. (C2H302)Cs; [3396-11-0] VARIABLES: PREPARED BY: Temperature. Baldini, P. EXPERIMENTAL VALUES: 228 501 40 242 515 45 249 522 50 250 256b 529 58 257 530 60 ,f 247 520 65 , 235 508 70 , 221 494 75 196 469 80 200 167 440 85 __ ..!!.2. 174 447 90 178 451 95 181 454 100 E a T/K values· calculated by the compiler. 50 lOOx 100 b 456 °c in the original table (compiler). 2 (C H 0 1Cs 2 3 2 Characteristic point(s): Eutectic, E, at 167 °c (164 °c according to Fig. 9 of the original paper; compiler) and 10Ox2= 85 (authors). Intermediate compound(s): (C2H302)7Cd2Cs3' congruently melting at 257 °c (255 °c, thermographic analysis), and exhibiting a polymorphic transition (at 130 °c, thermographic analysis; 133 °C, conductometry). Note - The system was investigated at 401~ lO0x2 ~ 100. AUXILIARY INFORMATION METHOD/APPARATUS/PROCEDURE: SOURCE AND PURITY OF MATERIALS: Visual polythermal method; temperatures Not stated. measured with a Chromel-Alumel thermocouple and a PP potentiometer. Additional investigations were performed by means of thermographical analysis, electrical conductometry, and X-ray diffractometry. NOTE: The occurrence of the intermediate compound is supported by X-ray diffractometry, and seems reliable. According to the authors~ this compound has a density of 2.472 g cm- • ESTIMATED ERROR: Although the Tfus(2) value (454 K) given in this paper is lower than the corresponding Temperature: accuracy probably +2 K one from Table 1 of the Preface, i.e., (compiler) • 463 K, the general trend of the phase diagram should be considered as REFERENCES: substantially correct. -

Crystal Structure Transformations in Binary Halides
1 A UNITED STATES DEPARTMENT OF A111D3 074^50 IMMERCE JBLICAT10N NSRDS—NBS 41 HT°r /V\t Co^ NSRDS r #C£ DM* ' Crystal Structure Transformations in Binary Halides u.s. ARTMENT OF COMMERCE National Bureau of -QC*-| 100 US73 ho . 4 1^ 72. NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation’s science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation’s physical measure- ment system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scien- tific community, industry, and commerce. The Institute consists of a Center for Radia- tion Research, an Office of Measurement Services and the following divisions: Applied Mathematics—Electricity—Heat—Mechanics—Optical Physics—Linac Radiation 2—Nuclear Radiation 2—Applied Radiation 2—Quantum Electronics 3— Electromagnetics 3—Time and Frequency 3—Laboratory Astrophysics 3—Cryo- 3 genics .