Gall Structure and Specificity in Bostrychia Culture Isolates (Rhodomelaceae, Rhodophyta)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Official Club Brochure 2018 – 2019
OFFICIAL CLUB BROCHURE 2018 – 2019 ROYAL MOTOR YACHT CLUB OF NSW PORT HACKING BRANCH Contents Welcome............................................... 1 A Brief History...................................... 3 Clubhouse - An Experience to Savour................ 6 - Dining at RMYC - Port Hacking..... 9 - Social Activities............................... 13 Waterfront Facilities............................ 17 Becoming a Member........................... 24 Member Benefits................................. 29 Sailing Division..................................... 33 Cruising................................................. 44 RMYC Golf Club................................... 45 Fishing Club......................................... 49 Port Hacking Game Fishing Club...... 52 How to Find Us.................................... 55 PREMIER PUBLISHING Royal Motor Yacht Club Port Hacking and Premier wish to thank 14 Ellis St the advertisers who appear in this publication for their support and South Yarra VIC 3141 wish them every business success. The contents of this brochure are believed to be correct at the time of printing, nevertheless, T 03 9521 7994 Royal Motor Yacht Club Port Hacking we cannot endorse and readers should not rely solely upon the E [email protected] accuracy of any statements or claims contained herein without W www.premierpublishing.com.au prior consultation with the service provider. Welcome to ROYAL MOTOR YACHT CLUB PORT HACKING We look forward to welcoming you to our club. ocated on Port Hacking, the Royal Motor of on-water interests. There are groups who Enjoy a family meal in Yachties Bistro, open into the Club to ensure we continue to grow and Yacht Club - Port Hacking is a recreational, enjoy Sailing, Cruising and Fishing. Our members seven days a week. Or come along to one of the evolve. With our stunning waterfront location, entertainment,L sailing and cruising club that is have also formed a long-running Social Golf Club many family-oriented social occasions. -
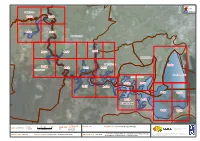
Appendix 3 – Maps Part 5
LEGEND LGAs Study area FAIRFIELD LGA ¹ 8.12a 8.12b 8.12c 8.12d BANKSTOWN LGA 8.12e 8.12f 8.12i ROCKDALE LGA HURSTVILLE LGA 8.12v 8.12g 8.12h 8.12j 8.12k LIVERPOOL LGA NORTH BOTANY BAY CITY OF KOGARAH 8.12n 8.12o 8.12l 8.12m 8.12r 8.12s 8.12p 8.12q SUTHERLAND SHIRE 8.12t 8.12u COORDINATE SCALE 0500 1,000 2,000 PAGE SIZE FIG NO. 8.12 FIGURE TITLE Overview of Site Specific Maps DATE 17/08/2010 SYSTEM 1:70,000 A3 © SMEC Australia Pty Ltd 2010. Meters MGA Z56 All Rights Reserved Data Source - Vegetation: The Native Vegetation of the Sydney Metropolitan Catchment LOCATION I:Projects\3001765 - Georges River Estuary Process Management Authority Area (Draft) (2009). NSW Department of Environment, Climate Change PROJECT NO. 3001765 PROJECT TITLE Georges River Estuary Process Study CREATED BY C. Thompson Study\009 DATA\GIS\ArcView Files\Working files and Water. Hurstville, NSW Australia. LEGEND Weed Hotspot Priority Areas Study Area LGAs Riparian Vegetation & EEC (Moderate Priority) Riparian Vegetation & EEC (High Priority) ¹ Seagrass (High Priority) Saltmarsh (High Priority) Estuarine Reedland (Moderate Priority) Mangrove (Moderate Priority) Swamp Oak (Moderate Priority) Mooring Areas River Area Reserves River Access Cherrybrook Park Area could be used for educational purposes due to high public usage of the wharf and boat launch facilities. Educate on responsible use of watercraft, value of estuarine and foreshore vegetation and causes and outcomes of foreshore FAIRFIELD LGA erosion. River Flat Eucalypt Forest Cabramatta Creek (Liverpool LGA) - WEED HOT SPOT Dominated by Balloon Vine (Cardiospermum grandiflorum) and River Flat Eucalypt Forest Wild Tobacco Bush (Solanum mauritianum). -

Sydney Impact Report: Residential Development Market March 2015
Sydney Impact Report Residential Development Market 2015 March Quarter Update The only way is up INSIDE THIS ISSUE: The previous March 2015 quarter has revealed that Sydney and its suburbs are slowly Residential LGA Zones 2 transforming into mid-high rise cities. During the quarter the Sydney metropolitan residential property value has jumped up to 30% in comparison to last year’s results. The low interest rate environment was one of the factors in pushing property value up. Residential Market 3 The low interest rates and a long running hot residential property market has seen many off-the-plan apartment sales sell out in record time. The high volume of sales Residential Development Supply 5 result may be a further incentive for developers to actively purchase and submit development approvals, and take advantage of the low interest rate. The Inner Sydney region, within 10 km proximity to the Sydney CBD, is set to become busier Market Observations 7 with many major developments flagged for construction during 2015. Residential development sites are popular to both local and foreign developers. The Economic Fundamentals 8 Inner Sydney sites were hotly contested for by both local and foreign developers whilst Middle and Outer Sydney sites (20km—30km to the Sydney CBD) were mostly acquired by local developers. Many of the recorded development sites in Inner and Middle Sydney were previously industrial sites. About Preston Rowe Paterson 10 Contact Us 12 Phone: +61 2 9292 7400 1 Fax: +61 2 9292 7404 Address: Level 14, 347 Kent Street Sydney NSW 2000 Email: [email protected] Follow us: Visit www.prpsydney.com.au © Copyright Preston Rowe Paterson NSW Pty Limited Residential LGA Zones According to the Real Estate Institute of Australia, the local government areas (LGAs) in the Sydney’s Statistical Division will be divided up into three geographical rings being inner, middle and outer. -

Towra Point Nature Reserve Ramsar Site: Ecological Character Description in Good Faith, Exercising All Due Care and Attention
Towra Point Nature Reserve Ramsar site Ecological character description Disclaimer The Department of Environment, Climate Change and Water NSW (DECCW) has compiled the Towra Point Nature Reserve Ramsar site: Ecological character description in good faith, exercising all due care and attention. DECCW does not accept responsibility for any inaccurate or incomplete information supplied by third parties. No representation is made about the accuracy, completeness or suitability of the information in this publication for any particular purpose. Readers should seek appropriate advice about the suitability of the information to their needs. The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the Australian Government or of the Minister for Environment Protection, Heritage and the Arts. Acknowledgements Phil Straw, Australasian Wader Studies Group; Bob Creese, Bruce Pease, Trudy Walford and Rob Williams, Department of Primary Industries (NSW); Simon Annabel and Rob Lea, NSW Maritime; Geoff Doret, Ian Drinnan and Brendan Graham, Sutherland Shire Council; John Dahlenburg, Sydney Metropolitan Catchment Management Authority. Symbols for conceptual diagrams are courtesy of the Integration and Application Network (ian.umces.edu/symbols), University of Maryland Center for Environmental Science. This publication has been prepared with funding provided by the Australian Government to the Sydney Metropolitan Catchment Management Authority through the Coastal Catchments Initiative Program. © State of NSW, Department of Environment, Climate Change and Water NSW, and Sydney Metropolitan Catchment Management Authority DECCW and SMCMA are pleased to allow the reproduction of material from this publication on the condition that the source, publisher and authorship are appropriately acknowledged. -

NSW Sydney Moorings Analysis
NSW Sydney Moorings Analysis Sydney Harbour (Area A) Date Last Mooring Total No. of No. of Private Applicant Mooring Bay Code/Bay Name Allocated & Ratio Applicants Moorings Totals Totals Comments AA - DARLING POINT 4 5/06/2015 25 AC - DOUBLE BAY 31 21/05/2015 203 AD - ELIZABETH BAY 32 9/04/2015 20 AE - FELIX BAY 11 30/03/2015 39 AF - HERMIT BAY 8 31/05/2015 30 AJ - PARSLEY BAY 13 28/05/2015 44 AO - ROSE BAY 40 2/06/2015 138 AP - RUSHCUTTERS BAY 58 27/04/2015 75 AT - VAUCLUSE BAY 25 28/05/2015 72 34% AU - WATSONS BAY 45 5/03/2015 131 267 777 Sydney Harbour (Area B) BB - BEAUTY POINT 0 8/05/2014 17 BE - CASTLECRAG 5 12/05/2015 45 BN - KILLARNEY 14 2/06/2015 68 BP - LONG BAY 5 29/04/2015 86 BR - NORTHBRIDGE 0 23/03/2015 52 BS - PEARL BAY 1 19/05/2015 145 BT - PICKERING POINT 0 7/05/2015 56 BU - POWDER HULK BAY 6 25/05/2015 105 BV - QUAKERS HAT BAY 0 12/06/2015 93 BX - SAILORS BAY 12 8/04/2015 62 BY - SALT PAN CREEK 3 7/05/2015 25 CA - SEAFORTH 0 28/05/2015 56 CD - SUGARLOAF BAY 1 29/04/2015 26 CE - SUGARLOAF POINT 8 7/02/2015 28 7% CH - WILLOUGHBY BAY 5 10/09/2014 32 60 896 Sydney Harbour (Area C) AG - LITTLE MANLY COVE 24 6/05/2015 35 AH - MANLY COVE 52 30/01/2015 86 AI - NORTH HARBOUR 106 1/04/2015 111 BA - BALMORAL 36 2/12/2014 56 BL - FISHER BAY 15 16/02/2015 18 BZ - SANDY BAY 22 17/02/2014 24 GE - CAREENING COVE 27 9/02/2009 18 GL - KIRRIBILLI 8 19/08/1992 2 GN - LITTLE SIRIUS COVE 10 9/04/2015 80 GP - MOSMAN BAY 48 29/01/2015 90 GQ - NEUTRAL BAY 50 6/01/2015 47 GU - SHELL COVE 30 2/06/2015 74 65% GW - TAYLORS BAY 0 28/05/2015 21 428 -

Hawkesbury Shelf Environmental Background Report
HAWKESBURY SHELF MARINE BIOREGION ASSESSMENT Hawkesbury Shelf environmental background report Background The NSW Marine Estate Management Authority (the Authority) was established by the NSW Government in 2013 to advise on policies, priorities and directions for the NSW marine estate. The NSW marine estate includes marine waters, estuaries and the coast. It extends seaward out to three nautical miles and from the Queensland border in the north to the Victorian border in the south. The full definition and map can be found at www.marine.nsw.gov.au. Contributors The Authority acknowledges the key contributions of officers from the following in preparing this report: • NSW Department of Primary Industries • Office of Environment and Heritage • Transport for NSW • Department of Planning and Environment • Marine Estate Expert Knowledge Panel Published by the NSW Marine Estate Management Authority Hawkesbury Shelf marine bioregion assessment – Hawkesbury Environmental background report First published February 2016 ISBN 978-1-74256-893-5 More information This paper and more information about the Hawkesbury Shelf marine bioregion assessment are available at www.marine.nsw.gov.au. RM8 reference INT15/135530 © State of New South Wales through the Department of Industry, Skills and Regional Development, 2016.This publication is copyright. You may download, display, print and reproduce this material provided that the wording is reproduced exactly, the source is acknowledged, and the copyright, update address and disclaimer notice are retained. To copy, adapt, publish, distribute or commercialise any of this publication you will need to seek permission from the Department of Industry, Skills and Regional Development. Disclaimer: The information contained in this publication is based on knowledge and understanding at the time of writing (February 2016). -

Analysis of the Mycosporine-Like Amino Acid (MAA) Pattern of the Salt Marsh Red Alga Bostrychia Scorpioides
marine drugs Article Analysis of the Mycosporine-Like Amino Acid (MAA) Pattern of the Salt Marsh Red Alga Bostrychia scorpioides Maria Orfanoudaki 1 , Anja Hartmann 1,*, Julia Mayr 1,Félix L. Figueroa 2 , Julia Vega 2 , John West 3, Ricardo Bermejo 4 , Christine Maggs 5 and Markus Ganzera 1 1 Institute of Pharmacy/Pharmacognosy, University of Innsbruck, Innrain 80-82, 6020 Innsbruck, Austria; [email protected] (M.O.); [email protected] (J.M.); [email protected] (M.G.) 2 Experimental Centre Grice-Hutchinson, Institute of Blue Biotechnology and Development (IBYDA), University of Malaga, 29004 Malaga, Spain; [email protected] (F.L.F.); [email protected] (J.V.) 3 School of BioSciences, University of Melbourne, Parkville, VIC 3010, Australia; [email protected] 4 Earth and Ocean Sciences, School of Natural Sciences and Ryan Institute, National University of Ireland, H91 TK33 Galway, Ireland; [email protected] 5 Medical Biology Centre, School of Biological Sciences, Queen’s University Belfast, Belfast BT22 1PF, UK; [email protected] * Correspondence: [email protected]; Tel.: +43-512-507-58430 Abstract: This study presents the validation of a high-performance liquid chromatography diode array detector (HPLC-DAD) method for the determination of different mycosporine-like amino acids (MAAs) in the red alga Bostrychia scorpioides. The investigated MAAs, named bostrychines, have only been found in this specific species so far. The developed HPLC-DAD method was successfully applied for the quantification of the major MAAs in Bostrychia scorpioides extracts, collected from four Citation: Orfanoudaki, M.; different countries in Europe showing only minor differences between the investigated samples. -

Emergency Survey of Pacific Oysters
Postprint This is an Accepted Manuscript of an article published in [Aquaculture] available online 17 December 2013 at http://www.sciencedirect.com/science/article/pii/S0044848613006480 Please cite as: Paul-Pont, I., Evans, O., Dhand, N. K., Rubio, A., Coad, P., & Whittington, R. J. (2014). Descriptive epidemiology of mass mortality due to Ostreid herpesvirus-1 (OsHV-1) in commercially farmed Pacific oysters (Crassostrea gigas) in the Hawkesbury River estuary, Australia. Aquaculture, 422-423, 146-159. Descriptive epidemiology of mass mortality due to Ostreid herpesvirus-1 (OsHV-1) in commercially farmed Pacific oysters (Crassostrea gigas) in the Hawkesbury River estuary, Australia I Paul-Pont1, O Evans1, NK Dhand1, A Rubio2, P Coad2 and RJ Whittington1 1Faculty of Veterinary Science, University of Sydney, 425 Werombi Road, Camden NSW 2570, Australia 2Hornsby Shire Council, 296 Pacific Highway, PO Box 37, Hornsby NSW 1630, Australia Corresponding author: RJ Whittington e-mail [email protected]; tel +61 2 93511619; fax +61 2 93511618 Abstract Mortality of farmed triploid Pacific oysters (Crassostrea gigas) associated with Ostreid herpesvirus-1 (OsHV-1) was first recorded in Australia in the Georges River/Botany Bay estuary (New South Wales) in late 2010. Two years later, the first sign of possible inter-estuarine spread was observed when commercial triploid Pacific oysters in the Hawkesbury River estuary, located 50 km north of Botany Bay, were affected by mass mortality. The aim of this study was to describe the epidemiological -

2017 Gymea Bay Public School Annual Report
Gymea Bay Public School Annual Report 2017 2116 Page 1 of 25 Gymea Bay Public School 2116 (2017) Printed on: 5 April, 2018 Introduction The Annual Report for 2017 is provided to the community of Gymea Bay as an account of the school's operations and achievements throughout the year. It provides a detailed account of the progress the school has made to provide high quality educational opportunities for all students, as set out in the school plan. It outlines the findings from self–assessment that reflect the impact of key school strategies for improved learning and the benefit to all students from the expenditure of resources, including equity funding. Michelle Michael Relieving Principal School contact details Gymea Bay Public School Gymea Bay Rd Gymea Bay, 2227 www.gymeabay-p.schools.nsw.edu.au [email protected] 9524 6852 Message from the Principal Gymea Bay Public School is an outstanding school with high expectations achieved for our motivated students through the strong partnership our school and teachers have with the parents and the wider community. All stakeholders work collaboratively and harmoniously together ensuring the best possible outcome for our students. Students have continued to participate and excel in a wide range of learning programs, team and individual sports and curriculum enrichment activities. the school's reputation for academic performance and delivery of a broad curriculum has been sustained and enhanced. Opportunities for students to enjoy and experience triumphs in cultural, artistic, environmental and sporting areas are many, and this report highlights a number of these achievements over 2017. -

Organellar Genome Evolution in Red Algal Parasites: Differences in Adelpho- and Alloparasites
University of Rhode Island DigitalCommons@URI Open Access Dissertations 2017 Organellar Genome Evolution in Red Algal Parasites: Differences in Adelpho- and Alloparasites Eric Salomaki University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/oa_diss Recommended Citation Salomaki, Eric, "Organellar Genome Evolution in Red Algal Parasites: Differences in Adelpho- and Alloparasites" (2017). Open Access Dissertations. Paper 614. https://digitalcommons.uri.edu/oa_diss/614 This Dissertation is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Dissertations by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. ORGANELLAR GENOME EVOLUTION IN RED ALGAL PARASITES: DIFFERENCES IN ADELPHO- AND ALLOPARASITES BY ERIC SALOMAKI A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGICAL SCIENCES UNIVERSITY OF RHODE ISLAND 2017 DOCTOR OF PHILOSOPHY DISSERTATION OF ERIC SALOMAKI APPROVED: Dissertation Committee: Major Professor Christopher E. Lane Jason Kolbe Tatiana Rynearson Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2017 ABSTRACT Parasitism is a common life strategy throughout the eukaryotic tree of life. Many devastating human pathogens, including the causative agents of malaria and toxoplasmosis, have evolved from a photosynthetic ancestor. However, how an organism transitions from a photosynthetic to a parasitic life history strategy remains mostly unknown. Parasites have independently evolved dozens of times throughout the Florideophyceae (Rhodophyta), and often infect close relatives. This framework enables direct comparisons between autotrophs and parasites to investigate the early stages of parasite evolution. -

Rhodophyta, Rhodomelaceae): an Important Systematic Character by Celia M
ATOLL RESEARCH BULLETIN NO. 312 PROCARP STRUCI'URE IN SOME CARIBBEAN SPECIES OF BOSTRYCHLA MONTAGNE (RHODOPHYTA, RHODOMELACEAE): AN IMPORTANT SYSTEMATIC CHARACTER BY CELIA M. SMITH, AND JAMES N. NORRIS ISSUED BY NATIONAL MUSEUM OF NATUFtAL HISTORY SMITHSONIAN INSTITUTION W&HINGTON,D.C.,U.SA. October 1988 PROCARP STRUCTURE IN SOME CARIBBEAN SPECIES OF BOSTRYCHU MONTAGNE (RHODOPHYTA, RHODOMELACEAE): - AN IMPORTANT SYSTEMATIC CHARACTER ABSTRACT Newly elucidated features which can be used for taxonomic purposes are shown by the pre- fertilization procarp structure in species of the red alga Bostrychia Mont. Because female gametophytes are apparently rare in Caribbean field populations, having been seldom collected, these reproductive characteristics have not been evaluated previously, but may provide greater stability to the systematics of Bosbychia which is now based almost entirely on vegetative characteristics. Four species of Bosbychia, as known from the Caribbean, B. montagnei Harv., B. binden Harv., B. tenella (Lamour.) J. Ag., and B. mdicans f. moniliformis Post, and one taxon of uncertain status, B. sp.?, were grown in culture and showed species specific features. Pre-fertilization structures that were quantified revealed differences in the length and width of trichogynes, the distribution and number of procarps present in fertile regions of the branchlets, and the number of cells of the fertile region. These differences show that reproductive structures are important taxonomic characters for species of Bosbychia and support the suggestion that Bostrychia is a primitive genus in the Rhodomelaceae. INTRODUCTION The red algal genus Bostrychia Montagne (1842, p.39) (Ceramiales, Rhodomelaceae) is wide- spread, occurring from tropical to cool-temperate regions, and usually associated with mangroves. -

The Great Kai'mia
The Great Kai’Mia Way “You cannot teach a land ethic... people learn by being involved with nature” - Anon The Great Kai’Mia Way The aim of the Great Kai'mia Way project is to create a network of way-marked routes for informal recreation linking river foreshores, parks, bushland reserves, public transport and other facilities in the Georges River region, by making connections between existing tracks and trails; encouraging this and future generations of people to explore and experience: • The rich diversity of wildlife and plants • Rivers waterways and catchment processes • A wealth of Aboriginal heritage • Stories of the area's colourful history 2 Table of Contents Forward ....................................................................................................................................... 8 Executive Summary .................................................................................................................... 10 Introduction .............................................................................................................................. 16 Report Structure ............................................................................................................................... 17 Aims of this report ............................................................................................................................ 18 Chapter One .............................................................................................................................. 19 Background ......................................................................................................................................