Hawkesbury Shelf Environmental Background Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Nsw Rail Timetables Rail and Tram News
AUSTRALASIAN TIMETABLE NEWS No. 268, December 2014 ISSN 1038-3697 RRP $4.95 Published by the Australian Timetable Association www.austta.org.au NEW NSW RAIL TIMETABLES designated as Hamilton Yard (Hamilton Station end) and Sydney area Passenger WTT 15 Nov 2014 Hamilton Sidings (Buffer Stop end). Transport for NSW has published a new Passenger Working Timetable for the Sydney area, version 3.70. Book 2 The following sections of the Working Timetable will be re- Weekends is valid from 15 November, and Book 1 issued with effect from Saturday 3 January 2015: • Weekdays valid from 17 November. There appear to be no Section 7- Central to Hornsby-Berowra (All Routes) significant alterations other than the opening of Shellharbour • Section 8- City to Gosford-Wyong-Morisset- Junction station closing of Dunmore station. A PDF of the Broadmeadow-Hamilton new South Coast line Public timetable can be accessed from • Section 9- Hamilton to Maitland-Dungog/Scone. the Sydney trains website. Cover pages, Explanatory Notes and Section Maps will also be issued. Additionally, amendments to Section 6 will need Sydney area Freight WTT 15 Nov 2014 to be made manually to include updated run numbers and Transport for NSW has published a new Freight Working changes to Sydney Yard working as per Special Train Notice Timetable for the Sydney area, version 3.50. Book 5 0034-2015. The re-issued sections of Books 1 & 2 will be Weekends is valid from 15 November, and Book 4 designated as Version 3.92, and replace the corresponding Weekdays valid from 17 November. There appear to be no sections of Working Timetable 2013, Version 3.31, reprint significant alterations. -

Official Club Brochure 2018 – 2019
OFFICIAL CLUB BROCHURE 2018 – 2019 ROYAL MOTOR YACHT CLUB OF NSW PORT HACKING BRANCH Contents Welcome............................................... 1 A Brief History...................................... 3 Clubhouse - An Experience to Savour................ 6 - Dining at RMYC - Port Hacking..... 9 - Social Activities............................... 13 Waterfront Facilities............................ 17 Becoming a Member........................... 24 Member Benefits................................. 29 Sailing Division..................................... 33 Cruising................................................. 44 RMYC Golf Club................................... 45 Fishing Club......................................... 49 Port Hacking Game Fishing Club...... 52 How to Find Us.................................... 55 PREMIER PUBLISHING Royal Motor Yacht Club Port Hacking and Premier wish to thank 14 Ellis St the advertisers who appear in this publication for their support and South Yarra VIC 3141 wish them every business success. The contents of this brochure are believed to be correct at the time of printing, nevertheless, T 03 9521 7994 Royal Motor Yacht Club Port Hacking we cannot endorse and readers should not rely solely upon the E [email protected] accuracy of any statements or claims contained herein without W www.premierpublishing.com.au prior consultation with the service provider. Welcome to ROYAL MOTOR YACHT CLUB PORT HACKING We look forward to welcoming you to our club. ocated on Port Hacking, the Royal Motor of on-water interests. There are groups who Enjoy a family meal in Yachties Bistro, open into the Club to ensure we continue to grow and Yacht Club - Port Hacking is a recreational, enjoy Sailing, Cruising and Fishing. Our members seven days a week. Or come along to one of the evolve. With our stunning waterfront location, entertainment,L sailing and cruising club that is have also formed a long-running Social Golf Club many family-oriented social occasions. -

Lenten Program 2021
The Community of the Church of Broken Bay 1 Diocese of Broken Bay - Lenten Program 2021 Reflecting Together on Bishop Anthony’s Pastoral Letter “Jesus Christ, our Beginning and End” Jesus Christ, the Alpha and the Omega 2 Reflecting Together on Bishop Anthony’s Pastoral Letter The Community of the Church of Broken Bay 3 Jesus Christ, the Alpha and the Omega Diocese of Broken Bay - Lenten Program 2021 Jesus Christ, the Alpha and the Omega 4 Letten Program 2021 © 2021 Catholic Diocese of Broken Bay, Australia Reflecting TogetherTogether on on Bishop Bishop Anthony’s Anthony’swww.bbcatholic.org.au Pastoral Pastoral Letter Letter The Community of the Church of Broken Bay 5 Introduction - Bishop Anthony fter one year as your Bishop, it was a joy for me to be able to offer you my Pastoral Letter in November 2020, which presented the fruit of my A prayer and contemplation on the priorities that I would like us to embrace as the community of the Diocese of Broken Bay. Thank you for receiving these with an open heart, and in a particular way, thank you for gathering during this Lenten season to reflect further and consider how these priorities can be more intentionally lived in our own lives and within our communities. The beginning and the end of our contemplation is the person of Jesus Christ; our constant during change. Empowered and united by the Holy Spirit in our common faith, we are each called to continue to grow in holiness and be formed as disciples, discovering our own vocation arising from our baptism, and living an authentic Christian life that places justice and mercy at the heart. -

Adding Value and Adapting to Trends
TALKING SHOP Enjoying Harbour Bridge views and just minutes from the Sydney city centre, Rushcutters Bay has 107 berths and is described as the jewel in the d’Albora crown. Brett Bolton and operation of the marinas and for acquisition programmes that tendered offers on new properties. In 2011 Bolton was engaged by McGrathNicol to take Horizon Shores in Queensland, one of the largest marinas in Australia, out of receivership. “The marina has 500 wet, 350 dry slips, 200-trailer storage Adding value and and a haul out facility,” said Bolton. “It was a business with many moving parts and new challenges on a daily basis.” adapting to trends In July, 2014, he began his current role as CEO of d’Albora Marinas. Brett Bolton, CEO of d’Albora Marinas, heads up the largest marina group in D’Albora operates seven sites in and around Sydney and Melbourne. The Australia. Speaking to Robert Wilkes from his office at d’Albora headquarters company founder, Jeff d’Albora, sold in Sydney, Bolton discusses innovation, strategy, customer service and the property in 2000 to what was then branding. Macquarie Leisure and is now Ardent Brett Bolton has held a number headquartered in Brisbane. Ariadne Leisure Ltd, a billion dollar multifaceted of high profile management roles in owned four marinas in Queensland, recreation company listed on the some of the largest recreation and New South Wales and New Zealand, Australia Stock Exchange. In addition marina companies in Australia and including Orams Marine Village in to marinas, the company operates New Zealand. He started in the marine Auckland. -

Central Coast and Hawkesbury River Recreational Fishing Guide
Central Coast and Hawkesbury River Recreational Fishing Guide Fisheries Compliance Unit • fish aggregating devices (FADs) to enhance fishing for dolphinfish and even tuna and August 2020 marlin; Fishing is a fun, outdoor activity for the whole • creation of recreational fishing havens; family. Fishing rules help ensure healthy and sustainable fisheries for future generations. • angler facilities such as fish cleaning tables and fishing platforms; The Central Coast’s waterways provide excellent beach, rock, and boat fishing opportunities. This • stocking of freshwater fish in dams and rivers; guide provides essential information on fishing, • essential research on popular recreational fish including any closures and restrictions, which apply species; within the Central Coast district, extending from Munmorah State Recreation Park in the north, to • restoring important fish habitat; the southern bank of the Hawkesbury River. • marine stocking of prawns in estuaries; DPI fisheries officers routinely patrol waterways, • angler education and advisory programs such boat ramps and foreshores to advise anglers about as the Fishcare Volunteer program, fishing responsible fishing practices and to ensure workshops, Get Hooked…it's fun to fish compliance with NSW fishing regulations. primary schools education and fishing guides. Information on bag and size limits and legal fishing Much more information is available at gear can be obtained at www.dpi.nsw.gov.au/fisheries. www.dpi.nsw.gov.au/fisheries or by visiting your local DPI fisheries office. You can pay the NSW recreational fishing fee at www.onegov.nsw.gov.au or by calling 1300 369 To report suspected illegal fishing activity, call the 365 or at many outlets throughout NSW, such as Fishers Watch phone line on 1800 043 536 (free fishing tackle stores, caravan parks, local shops, call) or report on-line at service stations and many Kmart stores. -

EORA Mapping Aboriginal Sydney 1770–1850 Exhibition Guide
Sponsored by It is customary for some Indigenous communities not to mention names or reproduce images associated with the recently deceased. Members of these communities are respectfully advised that a number of people mentioned in writing or depicted in images in the following pages have passed away. Users are warned that there may be words and descriptions that might be culturally sensitive and not normally used in certain public or community contexts. In some circumstances, terms and annotations of the period in which a text was written may be considered Many treasures from the State Library’s inappropriate today. Indigenous collections are now online for the first time at <www.atmitchell.com>. A note on the text The spelling of Aboriginal words in historical Made possible through a partnership with documents is inconsistent, depending on how they were heard, interpreted and recorded by Europeans. Original spelling has been retained in quoted texts, while names and placenames have been standardised, based on the most common contemporary usage. State Library of New South Wales Macquarie Street Sydney NSW 2000 Telephone (02) 9273 1414 Facsimile (02) 9273 1255 TTY (02) 9273 1541 Email [email protected] www.sl.nsw.gov.au www.atmitchell.com Exhibition opening hours: 9 am to 5 pm weekdays, 11 am to 5 pm weekends Eora: Mapping Aboriginal Sydney 1770–1850 was presented at the State Library of New South Wales from 5 June to 13 August 2006. Curators: Keith Vincent Smith, Anthony (Ace) Bourke and, in the conceptual stages, by the late Michael -

12 Walks in the Southern Illawarra
the creek and to the lower falls is an easy grade then a steep path takes you to a view of the upper falls. (This sec on was 5 & 6. Barren Grounds Nature Reserve —Illawarra Lookout closed at me of wri ng). It's worth a visit just to enjoy the Adjacent to Budderoo NP, Barren Grounds is one of the few ambience of the rainforest, do some Lyrebird spo ng, check large areas of heathland on the south coast and also has out the visitors’ centre and have a picnic or visit the kiosk. stands of rainforest along the escarpment edge. These varied Park entry fees apply. habitats are home to rare or endangered plants and animals Length: Up to 4km return including the ground parrot, eastern bristlebird and ger Time: Up to 2 hrs plus picnic me quoll. Barren Grounds offers short and long walks on well- formed tracks to great vantage points. The walks are stunning Illawarra Branch| [email protected] Grade: Easy to hard in spring when many of the heath flowers such as boronia, Access: Off Jamberoo Mtn Road, west from Kiama www.npansw.org | Find us on Facebook epacris and, if you’re lucky, waratah, are in full bloom. 3. Macquarie Pass Na onal Park —Cascades 5. Illawarra Lookout 12 Walks in the At the base of the Macquarie Pass and at the edge of the na onal Follow Griffiths Trail from the north-eastern corner of the car park is a deligh ul family friendly walk to a cascading waterfall. park. A er about 1 km walking through forest and heath take Southern Illawarra The parking area is on the northern side of the Illawarra Highway a short path on the le signed to Illawarra Lookout. -
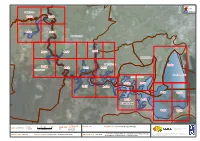
Appendix 3 – Maps Part 5
LEGEND LGAs Study area FAIRFIELD LGA ¹ 8.12a 8.12b 8.12c 8.12d BANKSTOWN LGA 8.12e 8.12f 8.12i ROCKDALE LGA HURSTVILLE LGA 8.12v 8.12g 8.12h 8.12j 8.12k LIVERPOOL LGA NORTH BOTANY BAY CITY OF KOGARAH 8.12n 8.12o 8.12l 8.12m 8.12r 8.12s 8.12p 8.12q SUTHERLAND SHIRE 8.12t 8.12u COORDINATE SCALE 0500 1,000 2,000 PAGE SIZE FIG NO. 8.12 FIGURE TITLE Overview of Site Specific Maps DATE 17/08/2010 SYSTEM 1:70,000 A3 © SMEC Australia Pty Ltd 2010. Meters MGA Z56 All Rights Reserved Data Source - Vegetation: The Native Vegetation of the Sydney Metropolitan Catchment LOCATION I:Projects\3001765 - Georges River Estuary Process Management Authority Area (Draft) (2009). NSW Department of Environment, Climate Change PROJECT NO. 3001765 PROJECT TITLE Georges River Estuary Process Study CREATED BY C. Thompson Study\009 DATA\GIS\ArcView Files\Working files and Water. Hurstville, NSW Australia. LEGEND Weed Hotspot Priority Areas Study Area LGAs Riparian Vegetation & EEC (Moderate Priority) Riparian Vegetation & EEC (High Priority) ¹ Seagrass (High Priority) Saltmarsh (High Priority) Estuarine Reedland (Moderate Priority) Mangrove (Moderate Priority) Swamp Oak (Moderate Priority) Mooring Areas River Area Reserves River Access Cherrybrook Park Area could be used for educational purposes due to high public usage of the wharf and boat launch facilities. Educate on responsible use of watercraft, value of estuarine and foreshore vegetation and causes and outcomes of foreshore FAIRFIELD LGA erosion. River Flat Eucalypt Forest Cabramatta Creek (Liverpool LGA) - WEED HOT SPOT Dominated by Balloon Vine (Cardiospermum grandiflorum) and River Flat Eucalypt Forest Wild Tobacco Bush (Solanum mauritianum). -

October 2020) ISSN 1836-599X
Issue 8 of 2020 (October 2020) ISSN 1836-599X PAINTED RIVER PROJECT By Asa Wahlquist The year six students at Forest Lodge have a tradition of leaving a gift for the students who follow them. The plan this year is for a mural featuring Johnstons Creek, but it is being thwarted by City of Sydney’s demand for $735 to lodge a Development Application. Forest Lodge principal Stephen Reed said the students wanted to do something on regeneration and sustainability. It is an ongoing theme, with a previous year instituting Waste-free Wednesdays. Mr Reed said the students were interested in regeneration in the wake of the summer bushfires, and the renaturalisation of Johnstons Creek caught their attention. Sydney Water is naturalising the Forest Lodge school students at work on the Painted River project (photo: Creek, replacing the concrete Asa Wahlquist) banks with sandstone set at different levels along the sides to create varied habitats, establishing a wetland, expanding the salt marsh and increasing the number and diversity of native plants along the sides. The students’ project is called The Painted River. Work began during Science Week, when the 36 students gathered by the Creek to study a display of water life put together by water ecologist Ian Wright and to paint what they saw under the guidance of artist Leo Robba. Dr Wright set up a table of samples collected from an undisturbed creek in Pittwater. The display included the stonefly, which he said is ‘supersensitive to changes in water quality.’ While he admits we will never know what Johnstons Creek was like before settlement, he thinks ‘some of these things could be brought back through naturalisation.’ Each of the students also completed a painted canvas. -

Commercial-In-Confidence
Any use of the Report, use of any part of it, or use of the names NewSouth Global, Expert Opinion Services, University of New South Wales, UNSW, the name of any unit of the University or the name of the Consultant, in direct or in indirect advertising or publicity, is forbidden. COMMERCIAL-IN-CONFIDENCE Report prepared on behalf of NSG Consulting A division of NewSouth Global Pty Limited Ecological issues in relation to BlueScope Steel SCP proposed salt water cooling for CH2M HILL Australia Pty Ltd by Dr Emma Johnston, Dr Jan Carey and Dr Nathan Knott August 2006 J069413 The University of New South Wales, Sydney 2052, DX 957 Sydney Ph: 1800 676 948 Fax: 1800 241 367 www.eos.unsw.edu.au Email: [email protected] CONTENTS Page Executive Summary: ................................................................................................ 1 Introduction............................................................................................................... 3 Predicted Changes in Temperature ........................................................................ 4 Temperature tolerances and preferences of organisms currently found in Port Kembla Harbour........................................................................................................ 9 General temperature effects on major biochemical processes...........................14 Species expected in a slightly to moderately disturbed estuarine system........15 Aspects of current environment that may be excluding species........................17 A review of the influences -

Thoracia, Balanomorpha, Tetraclitidae
Rei'. West. Aust. Mus. 1990, 14(4): 665-668 Occurrence of the barnacle Tesseropora rosea (Krauss) (Thoracica, Balanomorpha, Tetraclitidae) in western Australian waters. Diana S. Jones* Tesseropora rosea was originally described from one specimen collected at Algoa Bay, South Africa (Krauss 1848). Darwin (1854) recorded material from eastern Australia and, after examining the unique South African specimen commented (p. 335) 'There can be no doubt of the identify of the African and Australian specimens. It is a singular circumstance that the same species should occur in these two distant places, and, as far as at present known, not in the intermediate, more tropical coasts'. The species has not been collected either in South Africa, or on the 'intermediate more tropical coasts' since that time. However, as well as eastern Australia, the species is also known from Lord Howe Island and the Kermadec Islands (Endean et al. 1956a, 1956b; Foster 1978; Anderson & Anderson 1985). In eastern Australia T. rosea occurs between lat. 1905 and 380, and has never been recorded from western areas ofthe continent. The species occurs abundantly in exposed coastal areas and on flat rock platforms which are subjected to strong wave action, extending from betweenjust above mean low water neap to mean high water neap tidal levels (Pope 1945; Dakin et al. 1948, 1953; Denley & Underwood 1979; Anderson & Buckle 1983; Anderson & Anderson 1985). In 1986 three live specimens of T. rosea were collected on intertidal granitic rocks at Cottesloe, Western Australia, by Ms L. M. Marsh (WAM crustacean registration number WAM 2347-86). Since 1986 four isolated large individuals of T. -

Policy Analysis of Invasive Species Regulations in Sydney Harbour and San Francisco Bay
California State University, Monterey Bay Digital Commons @ CSUMB Capstone Projects and Master's Theses 2005 Policy analysis of invasive species regulations in Sydney Harbour and San Francisco Bay Alexis Radovich California State University, Monterey Bay Christina Schmunk California State University, Monterey Bay Follow this and additional works at: https://digitalcommons.csumb.edu/caps_thes Recommended Citation Radovich, Alexis and Schmunk, Christina, "Policy analysis of invasive species regulations in Sydney Harbour and San Francisco Bay" (2005). Capstone Projects and Master's Theses. 54. https://digitalcommons.csumb.edu/caps_thes/54 This Capstone Project is brought to you for free and open access by Digital Commons @ CSUMB. It has been accepted for inclusion in Capstone Projects and Master's Theses by an authorized administrator of Digital Commons @ CSUMB. Unless otherwise indicated, this project was conducted as practicum not subject to IRB review but conducted in keeping with applicable regulatory guidance for training purposes. For more information, please contact [email protected]. Policy Analysis of Invasive Species Regulations in Sydney Harbour and San Francisco Bay A Capstone Project Presented to the Faculty of Science and Environmental Policy in the College of Science, Media Arts, and Technology at California State University, Monterey Bay in Partial Fulfilment of the Requirements for the Degree of Bachelor of Science by Alexis Radovich Christina Schmunk December 15, 2005 Policy Analysis of Invasive Species in Sydney Harbour, Australia and San Francisco Bay, United States of America. By Alexis Radovich and Christina Schmunk The demand for the mass transportation of goods throughout the world is threatening the biodiversity and native habitats of local scale aquatic assemblages.