Emergency Survey of Pacific Oysters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Submission to Australia's Faunal Extinction Crisis Inquiry
Supplementary to my submission of the 5th September 2018 I would appreciate it if the panel would take the following information into account most of the local information has been taken from the web site: Campbelltown Koala Research and Database.com. which is Report undertaken by Prof. Robert Close and his team over a 27 year period.. www.campbelltown research and database.com 13th August 2019 Submission to Australia’s Faunal Extinction Crisis Inquiry Patricia Durman 28th July 2019 Thank you for allowing us to submit a submission into the state of koalas in NSW, my knowledge is mainly restricted to the local koala population and I have aimed this submission to the protection of these animals, but regard all native animals including koalas as part of the ecosystem that is required to keep the planet healthy. It is unfortunate that unless the panel has the power to delay development until they have made their report and it has been accepted by the government the Campbelltown koala population will have been seriously endangered by subdivision and the installation of new roads. I have referred to the koala population as the Campbelltown population, but this, of course, refers to all koalas living in the South West region of Sydney including Sutherland. HISTORY Although there were a few extinctions of native flora and fauna once the Aboriginal people had landed in Australia 40,000 years ago, perhaps even longer in the Cape York region, it wasn’t until Europeans arrived and cleared vast areas of bushland that the extinction of native animals escalated and in only 200 years we have become the continent with the most amount of extinction in the world. -

Official Club Brochure 2018 – 2019
OFFICIAL CLUB BROCHURE 2018 – 2019 ROYAL MOTOR YACHT CLUB OF NSW PORT HACKING BRANCH Contents Welcome............................................... 1 A Brief History...................................... 3 Clubhouse - An Experience to Savour................ 6 - Dining at RMYC - Port Hacking..... 9 - Social Activities............................... 13 Waterfront Facilities............................ 17 Becoming a Member........................... 24 Member Benefits................................. 29 Sailing Division..................................... 33 Cruising................................................. 44 RMYC Golf Club................................... 45 Fishing Club......................................... 49 Port Hacking Game Fishing Club...... 52 How to Find Us.................................... 55 PREMIER PUBLISHING Royal Motor Yacht Club Port Hacking and Premier wish to thank 14 Ellis St the advertisers who appear in this publication for their support and South Yarra VIC 3141 wish them every business success. The contents of this brochure are believed to be correct at the time of printing, nevertheless, T 03 9521 7994 Royal Motor Yacht Club Port Hacking we cannot endorse and readers should not rely solely upon the E [email protected] accuracy of any statements or claims contained herein without W www.premierpublishing.com.au prior consultation with the service provider. Welcome to ROYAL MOTOR YACHT CLUB PORT HACKING We look forward to welcoming you to our club. ocated on Port Hacking, the Royal Motor of on-water interests. There are groups who Enjoy a family meal in Yachties Bistro, open into the Club to ensure we continue to grow and Yacht Club - Port Hacking is a recreational, enjoy Sailing, Cruising and Fishing. Our members seven days a week. Or come along to one of the evolve. With our stunning waterfront location, entertainment,L sailing and cruising club that is have also formed a long-running Social Golf Club many family-oriented social occasions. -
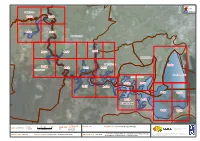
Appendix 3 – Maps Part 5
LEGEND LGAs Study area FAIRFIELD LGA ¹ 8.12a 8.12b 8.12c 8.12d BANKSTOWN LGA 8.12e 8.12f 8.12i ROCKDALE LGA HURSTVILLE LGA 8.12v 8.12g 8.12h 8.12j 8.12k LIVERPOOL LGA NORTH BOTANY BAY CITY OF KOGARAH 8.12n 8.12o 8.12l 8.12m 8.12r 8.12s 8.12p 8.12q SUTHERLAND SHIRE 8.12t 8.12u COORDINATE SCALE 0500 1,000 2,000 PAGE SIZE FIG NO. 8.12 FIGURE TITLE Overview of Site Specific Maps DATE 17/08/2010 SYSTEM 1:70,000 A3 © SMEC Australia Pty Ltd 2010. Meters MGA Z56 All Rights Reserved Data Source - Vegetation: The Native Vegetation of the Sydney Metropolitan Catchment LOCATION I:Projects\3001765 - Georges River Estuary Process Management Authority Area (Draft) (2009). NSW Department of Environment, Climate Change PROJECT NO. 3001765 PROJECT TITLE Georges River Estuary Process Study CREATED BY C. Thompson Study\009 DATA\GIS\ArcView Files\Working files and Water. Hurstville, NSW Australia. LEGEND Weed Hotspot Priority Areas Study Area LGAs Riparian Vegetation & EEC (Moderate Priority) Riparian Vegetation & EEC (High Priority) ¹ Seagrass (High Priority) Saltmarsh (High Priority) Estuarine Reedland (Moderate Priority) Mangrove (Moderate Priority) Swamp Oak (Moderate Priority) Mooring Areas River Area Reserves River Access Cherrybrook Park Area could be used for educational purposes due to high public usage of the wharf and boat launch facilities. Educate on responsible use of watercraft, value of estuarine and foreshore vegetation and causes and outcomes of foreshore FAIRFIELD LGA erosion. River Flat Eucalypt Forest Cabramatta Creek (Liverpool LGA) - WEED HOT SPOT Dominated by Balloon Vine (Cardiospermum grandiflorum) and River Flat Eucalypt Forest Wild Tobacco Bush (Solanum mauritianum). -

Output Chunks
MooneyMooney MooneyMooney CreekCreek TrackheadTrackhead toto SomersbySomersby This enjoyable walk starts from where the old Length: 16.1 km Pacific Hwy where you walk along dirt roads and trails for while alongside the wide Mooney Time: 6 hrs Mooney Creek, and under the huge F3 Mooney Climb: 680 m Mooney bridge. The wide track continues upstream, passing a few campsites before crossing Style: One way the wide creek at a pleasant large rock platform. Rating: Track: Hard Not too long after crossing the creek you will pass the lower Mooney Mooney Dam where the old Where: 9.1 km W of Gosford trail leads you uphill past another campsite, a Transport: car bus quarry to the Somersby Reservoir. The track then leads past some rural properties and across the Visit www.wildwalks.com for more info delightful Robinson Creek among the Gymea Lilies before finishing with a section of road walking to the Somersby Store. Brisbane Water National Park Side trips and Alternate routes mentioned in these notes are not included in the tracks overall rating, distance or time estimate. The notes only describe the side trips and Alternate routes in one direction. Allow extra time for resting and exploring areas of interest. Please ensure you and your group are well prepared and equipped for all possible hazards and delays. The authors, staff and owners of wildwalks take care in preparing these notes but will not accept responsibility for any inconvenience, loss or injury sustained by using these notes or maps. Please take care and share your experience through the website. -

Macrobrachium Intermedium in Southeastern Australia: Spatial Heterogeneity and the Effects of Species of Seagrass
MARINE ECOLOGY PROGRESS SERIES Vol. 75: 239-249, 1991 Published September 11 Mar. Ecol. Prog. Ser. Demographic patterns of the palaemonid prawn Macrobrachium intermedium in southeastern Australia: spatial heterogeneity and the effects of species of seagrass Charles A. Gray* School of Biological Sciences, University of Sydney, 2006, NSW. Australia ABSTRACT. The effects of species of seagrass (Zostera capricorni and Posidonia australis) on spatial and temporal heterogeneity in the demography of estuarine populations of the palaemonid prawn Macrobrachium intermedium across 65 km of the Sydney region, southeastern Australia, were examined. Three estuaries were sampled in 1983 and 1984 to assess the magnitude of intra- and inter- estuary variability in demographic characteristics among populations. Species of seagrass had no effect on the demographic patterns of populations: differences in the magnitude and directions of change in abundances, recruitment, reproductive characteristics, size structures and growth were as great among populations within each species of seagrass as those between the 2 seagrasses Abiotic factors, such as the location of a meadow in relation to depth of water and distance offshore, and the interactions of these factors with recruiting larvae are hypothesised to have greater influence than the species of seagrass in determining the distribution and abundance of these prawns. Spatial and temporal heterogeneity in demography was similar across all spatial scales sampled: among meadows (50 m to 3 km apart) in an estuary and among meadows in all 3 estuaries (10 to 65 km apart). Variability in demographic processes among populations in the Sydney region was most likely due to stochastic factors extrinsic to the seagrasses then~selves.I conclude that the demography of seagrass-dwelling estuarine populations of M. -

“Are New Developments Cleaning up the Cooks River Or Creating More Problems?”
Capacity Building and training needs analysis: Stage 1 Report “Are new developments cleaning up the Cooks River or creating more problems?” FINAL Brian Keogh 24 June, 2016 Report Basis This report partially fulfils two Cobalt59 requirements: It provides a baseline evaluation of the capacity of the Cooks River councils within a critical systems area (planning assessment in relation to water management). It provides a training assessment that will assist in developing this capacity. Page 1 of 46 Contents 1. Executive Summary ....................................................................................................... 3 State Environment Protection Policies (SEPP) .................................................................. 3 Local Environment Plans (LEP) ......................................................................................... 3 Development Control Plans (DCP) .................................................................................... 4 Training Recommendations ............................................................................................... 7 2. Capacity Assessment – Systems ................................................................................... 9 3. Background .................................................................................................................. 11 4. Planning Overview ....................................................................................................... 13 5. NSW State Environment Protection Policies (SEPPs) ................................................. -

Sydney Impact Report: Residential Development Market March 2015
Sydney Impact Report Residential Development Market 2015 March Quarter Update The only way is up INSIDE THIS ISSUE: The previous March 2015 quarter has revealed that Sydney and its suburbs are slowly Residential LGA Zones 2 transforming into mid-high rise cities. During the quarter the Sydney metropolitan residential property value has jumped up to 30% in comparison to last year’s results. The low interest rate environment was one of the factors in pushing property value up. Residential Market 3 The low interest rates and a long running hot residential property market has seen many off-the-plan apartment sales sell out in record time. The high volume of sales Residential Development Supply 5 result may be a further incentive for developers to actively purchase and submit development approvals, and take advantage of the low interest rate. The Inner Sydney region, within 10 km proximity to the Sydney CBD, is set to become busier Market Observations 7 with many major developments flagged for construction during 2015. Residential development sites are popular to both local and foreign developers. The Economic Fundamentals 8 Inner Sydney sites were hotly contested for by both local and foreign developers whilst Middle and Outer Sydney sites (20km—30km to the Sydney CBD) were mostly acquired by local developers. Many of the recorded development sites in Inner and Middle Sydney were previously industrial sites. About Preston Rowe Paterson 10 Contact Us 12 Phone: +61 2 9292 7400 1 Fax: +61 2 9292 7404 Address: Level 14, 347 Kent Street Sydney NSW 2000 Email: [email protected] Follow us: Visit www.prpsydney.com.au © Copyright Preston Rowe Paterson NSW Pty Limited Residential LGA Zones According to the Real Estate Institute of Australia, the local government areas (LGAs) in the Sydney’s Statistical Division will be divided up into three geographical rings being inner, middle and outer. -

Jellyfish Catostylus Mosaicus (Rhizostomeae) in New South Wales, Australia
- MARINE ECOLOGY PROGRESS SERIES Vol. 196: 143-155,2000 Published April 18 Mar Ecol Prog Ser l Geographic separation of stocks of the edible jellyfish Catostylus mosaicus (Rhizostomeae) in New South Wales, Australia K. A. Pitt*, M. J. Kingsford School of Biological Sciences, Zoology Building, A08 University of Sydney, New South Wales 2006, Australia ABSTRACT: The population structure of the commercially harvested jellyfish Catostylus mosaicus (Scyphozoa, Rhizostomeae) was investigated in estuaries and bays in New South Wales, Australia. Variations in abundance and recruitment were studied in 6 estuaries separated by distances ranglng from 75 to 800 km. Patterns of abundance differed greatly among estuaries and the rank abundance among estuaries changed on 5 out of the 6 times sampling occurred. Great variation in the timing of recruitment was also observed among estuaries. Variations in abundance and recruitment were as extreme among nearby estuaries as distant ones. Broad scale sampling and detailed time series of abundance over a period of 2.7 yr at 2 locations showed no consistent seasonal trend in abundance at 1 location, but there was some indication of seasonality at the second location. At Botany Bay, the abun- dance of medusae increased with distance into the estuary and on 19 out of the 30 times sampling occurred medusae were found at sites adjacent to where rivers enter the bay. Medusae were found to be strong swimmers and this may aid medusae in maintaining themselves in the upper-reaches of estu- aries, where advection from an estuary is least likely. Variability in patterns of abundance and recruit- ment suggested regulation by processes occurring at the scale of individual estuaries and, combined with their relatively strong swimming ability, supported a model of population retention within estuar- ies. -

Towra Point Nature Reserve Ramsar Site: Ecological Character Description in Good Faith, Exercising All Due Care and Attention
Towra Point Nature Reserve Ramsar site Ecological character description Disclaimer The Department of Environment, Climate Change and Water NSW (DECCW) has compiled the Towra Point Nature Reserve Ramsar site: Ecological character description in good faith, exercising all due care and attention. DECCW does not accept responsibility for any inaccurate or incomplete information supplied by third parties. No representation is made about the accuracy, completeness or suitability of the information in this publication for any particular purpose. Readers should seek appropriate advice about the suitability of the information to their needs. The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the Australian Government or of the Minister for Environment Protection, Heritage and the Arts. Acknowledgements Phil Straw, Australasian Wader Studies Group; Bob Creese, Bruce Pease, Trudy Walford and Rob Williams, Department of Primary Industries (NSW); Simon Annabel and Rob Lea, NSW Maritime; Geoff Doret, Ian Drinnan and Brendan Graham, Sutherland Shire Council; John Dahlenburg, Sydney Metropolitan Catchment Management Authority. Symbols for conceptual diagrams are courtesy of the Integration and Application Network (ian.umces.edu/symbols), University of Maryland Center for Environmental Science. This publication has been prepared with funding provided by the Australian Government to the Sydney Metropolitan Catchment Management Authority through the Coastal Catchments Initiative Program. © State of NSW, Department of Environment, Climate Change and Water NSW, and Sydney Metropolitan Catchment Management Authority DECCW and SMCMA are pleased to allow the reproduction of material from this publication on the condition that the source, publisher and authorship are appropriately acknowledged. -

NSW Sydney Moorings Analysis
NSW Sydney Moorings Analysis Sydney Harbour (Area A) Date Last Mooring Total No. of No. of Private Applicant Mooring Bay Code/Bay Name Allocated & Ratio Applicants Moorings Totals Totals Comments AA - DARLING POINT 4 5/06/2015 25 AC - DOUBLE BAY 31 21/05/2015 203 AD - ELIZABETH BAY 32 9/04/2015 20 AE - FELIX BAY 11 30/03/2015 39 AF - HERMIT BAY 8 31/05/2015 30 AJ - PARSLEY BAY 13 28/05/2015 44 AO - ROSE BAY 40 2/06/2015 138 AP - RUSHCUTTERS BAY 58 27/04/2015 75 AT - VAUCLUSE BAY 25 28/05/2015 72 34% AU - WATSONS BAY 45 5/03/2015 131 267 777 Sydney Harbour (Area B) BB - BEAUTY POINT 0 8/05/2014 17 BE - CASTLECRAG 5 12/05/2015 45 BN - KILLARNEY 14 2/06/2015 68 BP - LONG BAY 5 29/04/2015 86 BR - NORTHBRIDGE 0 23/03/2015 52 BS - PEARL BAY 1 19/05/2015 145 BT - PICKERING POINT 0 7/05/2015 56 BU - POWDER HULK BAY 6 25/05/2015 105 BV - QUAKERS HAT BAY 0 12/06/2015 93 BX - SAILORS BAY 12 8/04/2015 62 BY - SALT PAN CREEK 3 7/05/2015 25 CA - SEAFORTH 0 28/05/2015 56 CD - SUGARLOAF BAY 1 29/04/2015 26 CE - SUGARLOAF POINT 8 7/02/2015 28 7% CH - WILLOUGHBY BAY 5 10/09/2014 32 60 896 Sydney Harbour (Area C) AG - LITTLE MANLY COVE 24 6/05/2015 35 AH - MANLY COVE 52 30/01/2015 86 AI - NORTH HARBOUR 106 1/04/2015 111 BA - BALMORAL 36 2/12/2014 56 BL - FISHER BAY 15 16/02/2015 18 BZ - SANDY BAY 22 17/02/2014 24 GE - CAREENING COVE 27 9/02/2009 18 GL - KIRRIBILLI 8 19/08/1992 2 GN - LITTLE SIRIUS COVE 10 9/04/2015 80 GP - MOSMAN BAY 48 29/01/2015 90 GQ - NEUTRAL BAY 50 6/01/2015 47 GU - SHELL COVE 30 2/06/2015 74 65% GW - TAYLORS BAY 0 28/05/2015 21 428 -

Coastal Storms in Nsw and Their Effect on the Coast
I I. COASTAL STORMS IN N.s.w. .: AUGUST & NOVEMBER 1986 .1 and Their Effect on the Coast I 'I I 1'1 ! I I I I I I I I I I , . < May 198,8 , 'I " I I : COASTAL STORMS· IN N.s.w. I AUGUST & NOVEMBER 1986 I and Their Effect on the Coast I I I I I 1: 1 I I I I I M.N. CLARKE M.G. GEARY I Chief Engineer Principal Engineer I Prepared by : University of N.S.W. Water Research Laboratory I bID IPTIID311IIcr: ~Th'¥@~ , I May 1988 ------..! Coaat a Rivera Branch I I I I CONTENTS LIST OF FIGURES ( ii ) I LIST OF TABLES (iv) 1. INTRODUCTION I 2. SUMMARY 2 3. ENVIRONMENTAL CONDITIONS 5 I 3.1 METEOROLOGICAL DATA 5 3.2 TIDE LEVELS 7 3.3 WAVE HEIGHTS AND PERIODS 10 I 3.4 STORM mSTORY - AUGUST 1985 TO AUGUST 1986 13 4. WAVE RUN-UP 15 I 4.1 MANLY WARRINGAH BEACHES 15 4.2 BATEMANS BAY BEACHES 15 I 4.3 THEORETICAL ASSESSMENT 16 4.4 COMPARISON OF MEASURED AND THEORETICAL RUN-UP LEVELS 23 5. BEACH SURVEYS 25 ·1 5.1 GOSFORD BEACHES 25 5.2 MANLY W ARRINGAH BEACHES 28 I 5.3 BATEMANS BAY BEACHES 29 6. OFFSHORE SURVEYS . 30 6.1 SURVEY METHODS 30 I 6.2 GOSFORD BEACHES 30 6.3 WARRINGAH BEACHES 31 I 6.4 ASSESSMENT OF ACCURACY OF OFFSHORE SURVEYS 32 7. OFFSHORE SEDIMENT SAMPLES - GOSFORD BEACHES 34 7.1 SAMPLE COLLECTION 34 I 7.2 SAMPLE CLASSIFICATION 34 , 7.3 SAMPLE DESCRIPTIONS 34 I 7.4 COMPARISON OF 1983/84 AND POST STORM OFFSHORE DATA 36 8. -
![An Account of the English Colony in New South Wales [Volume 1]](https://docslib.b-cdn.net/cover/2437/an-account-of-the-english-colony-in-new-south-wales-volume-1-822437.webp)
An Account of the English Colony in New South Wales [Volume 1]
An Account of the English Colony in New South Wales [Volume 1] With Remarks on the Dispositions, Customs, Manners &c. of the Native Inhabitants of that Country. To Which are Added, Some Particulars of New Zealand: Complied by Permission, From the Mss. of Lieutenant-Governor King Collins, David (1756-1810) A digital text sponsored by University of Sydney Library Sydney 2003 colacc1 http://purl.library.usyd.edu.au/setis/id/colacc1 © University of Sydney Library. The texts and images are not to be used for commercial purposes without permission Prepared from the print edition published by T. Cadell Jun. and W. Davies 1798 All quotation marks are retained as data. First Published: 1798 F263 Australian Etext Collections at Early Settlement prose nonfiction pre-1810 An Account of the English Colony in New South Wales [Volume 1] With Remarks on the Dispositions, Customs, Manners &c. of the Native Inhabitants of that Country. To Which are Added, Some Particulars of New Zealand: Complied by Permission, From the Mss. of Lieutenant-Governor King Contents. Introduction. SECT. PAGE I. TRANSPORTS hired to carry Convicts to Botany Bay. — The Sirius and the Supply i commissioned. — Preparations for sailing. — Tonnage of the Transports. — Numbers embarked. — Fleet sails. — Regulations on board the Transports. — Persons left behind. — Two Convicts punished on board the Sirius. — The Hyæna leaves the Fleet. — Arrival of the Fleet at Teneriffe. — Proceedings at that Island. — Some Particulars respecting the Town of Santa Cruz. — An Excursion made to Laguna. — A Convict escapes from one of the Transports, but is retaken. — Proceedings. — The Fleet leaves Teneriffe, and puts to Sea.