Final Contaminant Cadidate List 3 Microbes: Identifing the Universe
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bartonella Apis Sp. Nov., a Honey Bee Gut Symbiont of the Class Alphaproteobacteria
Serveur Academique´ Lausannois SERVAL serval.unil.ch Author Manuscript Faculty of Biology and Medicine Publication This paper has been peer-reviewed but does not include the final publisher proof-corrections or journal pagination. Published in final edited form as: Title: Bartonella apis sp. nov., a honey bee gut symbiont of the class Alphaproteobacteria. Authors: Keˇsnerov´aL, Moritz R, Engel P Journal: International journal of systematic and evolutionary microbiology Year: 2016 Jan Issue: 66 Volume: 1 Pages: 414-21 DOI: 10.1099/ijsem.0.000736 In the absence of a copyright statement, users should assume that standard copyright protection applies, unless the article contains an explicit statement to the contrary. In case of doubt, contact the journal publisher to verify the copyright status of an article. 1 Bartonella apis sp. nov., a honey bee gut symbiont of the 2 class Alphaproteobacteria 3 4 Lucie Kešnerová, Roxane Moritz, Philipp Engel* 5 6 Department of Fundamental Microbiology, University of Lausanne, CH-1015 7 Lausanne, Switzerland 8 9 Running title: Description of a bee gut symbiont 10 11 *Correspondence: 12 Prof. Philipp Engel 13 Department of Fundamental Microbiology 14 University of Lausanne, CH-1015 Lausanne, Switzerland 15 Tel.: +41 (0)21 692 56 12 16 e-mail: [email protected] 17 18 Category: New Taxa – Proteobacteria 19 Keywords: Apis mellifera; insect; Bartonella; gut microbiota; Alpha-1 20 21 Sequence deposition: The 16S rRNA gene sequences and protein-coding gene 22 sequences of the bacterial strains PEB0122T, PEB0149, PEB0150, BBC0104, and 23 BBC0108 from Apis mellifera, and the uncultured Rhizobiales bacterium from 24 Herpagnathos saltator are deposited in GenBank with accession numbers KP987849 25 – KP987886 and KT315729 – KT315734. -

Susceptibility and Resistance Data
toku-e logo For a complete list of references, please visit antibiotics.toku-e.com Imipenem Microorganism Genus, Species, and Strain (if shown) Concentration Range (μg/ml)Susceptibility and Achromobacter xylosoxidans subsp. denitrificans 0.25 - 4 Minimum Inhibitory Acinetobacter anitratus ≤0.008 128 Acinetobacter baumannii Concentration0.008 - 512 (MIC) Data Acinetobacter calcoaceticus 0.016 - >8 Issue date 01/06/2020 Acinetobacter haemolyticus ≤0.008 >16 Acinetobacter junii ≤0.12 >8 Acinetobacter lwoffii ≤0.008 >16 Acinetobacter spp. 0.008 - >64 Actinomyces gerencseriae ≤0.015 8 Actinomyces graevenitzii ≤0.015 0.25 Actinomyces israelii ≤0.015 8 Actinomyces meyeri ≤0.015 8 Actinomyces naeslundii 0.015 - 8 Actinomyces neuii ≤0.015 0.25 Actinomyces odontolyticus ≤0.015 8 Actinomyces radingae ≤0.015 0.25 Actinomyces schalii ≤0.015 0.25 Actinomyces spp. ≤0.008 8 Actinomyces turicensis ≤0.015 0.25 Actinomyces viscosus ≤0.015 0.5 Aerococcus spp. ≤0.008 4 Aerococcus urinae ≤0.008 4 Aeromonas caviae 0.25 - 4 Aeromonas hydrophila 0.25 - 16 Aeromonas spp. 0.12 - 4 Agrobacterium radiobacter 0.06 - 1 Alcaligenes faecalis 0.06 - >16 Alcaligenes odorans 0.25 - 1 Anaerococcus prevotii ≤0.016 0.25 Anaerococcus tetradius ≤0.016 0.03 Arcanobacterium pyogenes ≤0.03 0.25 Atopobium parvulum 0.25 Bacillus proteus 4 Bacillus spp. ≤0.008 4 Bacillus subtilis <0.025 Bacteroides caccae ≤0.06 8 Bacteroides capillosus 0.06 - 0.25 Bacteroides distasonis 0.03 - 8 Bacteroides eggerthii ≤0.125 0.5 Bacteroides fragilis ≤0.008 >128 Bacteroides fragilis gr. 0.03 - 4 Bacteroides levii 0.06 - 0.25 Bacteroides merdae ≤0.06 4 Bacteroides ovatus 0.03 - 16 Bacteroides splanchnicus 0.06 - 0.25 Bacteroides spp. -

Are You Suprised ?
B DAMB 721 Microbiology Final Exam B 100 points December 11, 2006 Your name (Print Clearly): _____________________________________________ I. Matching: The questions below consist of headings followed by a list of phrases. For each phrase select the heading that best describes that phrase. The headings may be used once, more than once or not at all. Mark the answer in Part 2 of your answer sheet. 1. capsid 7. CD4 2. Chlamydia pneumoniae 8. Enterococcus faecalis 3. oncogenic 9. hyaluronidase 4. pyruvate 10. interferon 5. Koplik’s spot 11. hydrophilic viruses 6. congenital Treponema pallidum 12. Streptococcus pyogenes 1. “spreading factor” produced by members of the staphylococci, streptococci and clostridia 2. viral protein coat 3. central intermediate in bacterial fermentation 4. persistant endodontic infections 5. a cause of atypical pneumonia 6. nonspecific defense against viral infection 7. has a rudimentary life cycle 8. HIV receptor 9. Hutchinson’s Triad 10. measles 11. resistant to disinfection 12. β-hemolytic, bacitracin sensitive, cause of suppurative pharyngitis 2 Matching (Continued): The questions below consist of diseases followed by a list of etiologic agents. Match each disease with the etiologic agent. Continue using Part 2 of your answer sheet. 1. dysentery 6. Legionnaire’s 2. botulism 7. gas gangrene 3. cholera 8. tuberculosis 4. diphtheria 9. necrotizing fascitis 5. enteric fever 10. pneumoniae/meningitis 13. Clostridium botulinum 14. Vibrio cholera 15. Mycobacterium bovis 16. Shigella species 17. Streptococcus pneumoniae 18. Clostridium perfringens 19. Salmonella typhi 20. Streptococcus pyogenes 3 II. Multiple Choice: Choose the ONE BEST answer. Mark the correct answer on Part 1 of the answer sheet. -

Pronunciation Guide to Microorganisms
Pronunciation Guide to Microorganisms This pronunciation guide is provided to aid each student in acquiring a greater ease in discussing, describing, and using specific microorganisms. Please note that genus and species names are italicized. If they cannot be italicized, then they should be underlined (example: a lab notebook). Prokaryotic Species Correct Pronunciation Acetobacter aceti a-se-toh-BAK-ter a-SET-i Acetobacter pasteurianus a-se-toh-BAK-ter PAS-ter-iann-us Acintobacter calcoacetius a-sin-ee-toe-BAK-ter kal-koh-a-SEE-tee-kus Aerococcus viridans (air-o)-KOK-kus vi-ree-DANS Agrobacterium tumefaciens ag-roh-bak-TEAR-ium too-me-FAY-she-ens Alcaligenes denitrificans al-KAHL-li-jen-eez dee-ni-TREE-fee-cans Alcaligenes faecalis al-KAHL-li-jen-eez fee-KAL-is Anabaena an-na-BEE-na Azotobacter vinelandii a-zoe-toe-BAK-ter vin-lan-DEE-i Bacillus anthracis bah-SIL-lus AN-thray-sis Bacillus lactosporus bah-SIL-lus LAK-toe-spore-us Bacillus megaterium bah-SIL-lus Meg-a-TEER-ee-um Bacillus subtilis bah-SIL-lus SA-til-us Borrelia recurrentis bore-RELL-ee-a re-kur-EN-tis Branhamella catarrhalis bran-hem-EL-ah cat-arr-RAH-lis Citrobacter freundii sit-roe-BACK-ter FROND-ee-i Clostridium perfringens klos-TREH-dee-um per-FRINGE-enz Clostridium sporogenes klos-TREH-dee-um spore-AH-gen-ease Clostridium tetani klos-TREH-dee-um TET-ann-ee Corynebacterium diphtheriae koh-RYNE-nee-back-teer-ee-um dif-THEE-ry-ee Corynebacterium hofmanni koh-RYNE-nee-back-teer-ee-um hoff-MAN-eye Corynebacterium xerosis koh-RYNE-nee-back-teer-ee-um zer-OH-sis Enterobacter -

View Tickborne Diseases Sample Report
1360 Bayport Ave, Ste B. San Carlos, CA 94070 1(866) 364-0963 | [email protected] | www. vibrant-wellness.com PATIENT PROVIDER NAME: DEMO REPORT GENDER: Male PRACTICE NAME: Vibrant IT4 Practice DATE OF BIRTH: 04/14/1998 AGE: 22 PROVIDER NAME: Demo Client, DDD (999994) ADDRESS: TEST STREET, TEST CITY, KY- 42437. ACCESSION ID: 2009220006 PHLEBOTOMIST: 607 SPECIMEN COLLECTION TIME: 09-21-2020 11:14 SPECIMEN RECEIVED TIME: 09-22-2020 05:14 FINAL REPORT TIME: 09-25-2020 15:56 FASTING: FASTING Your Vibrant Wellness TickBorne 2.0 panel results are enclosed. These results are intended to aid in the diagnosis of tickborne diseases by your healthcare provider. The Vibrant Tickborne Diseases panel tests for IgG and IgM antibodies for Borreliosis/Lyme disease as well as co-infection(s) and opportunistic infections with other tick-borne illnesses along with detection of DNA of the species causing these infections. The Vibrant Immunochip test is a semiquantitative assay that detects IgG and IgM antibodies in human serum. The PCR Test is a real-time PCR Assay designed for qualitative detection of infectious group- specific DNA in clinical samples. Interpretation of Report: The test results of antibody levels to the individual antigens are calculated by comparing the average intensity of the individual antibody to that of a reference population and cut-off chosen for each protein. Reference ranges have been established using a well characterized set of more than 300 serum samples and antibodies to specific bacteria tested. The results are displayed as In Control, Moderate, or High Risk.for each antigen tested. -

Genetic Diversity of Bartonella Species in Small Mammals in the Qaidam
www.nature.com/scientificreports OPEN Genetic diversity of Bartonella species in small mammals in the Qaidam Basin, western China Huaxiang Rao1, Shoujiang Li3, Liang Lu4, Rong Wang3, Xiuping Song4, Kai Sun5, Yan Shi3, Dongmei Li4* & Juan Yu2* Investigation of the prevalence and diversity of Bartonella infections in small mammals in the Qaidam Basin, western China, could provide a scientifc basis for the control and prevention of Bartonella infections in humans. Accordingly, in this study, small mammals were captured using snap traps in Wulan County and Ge’ermu City, Qaidam Basin, China. Spleen and brain tissues were collected and cultured to isolate Bartonella strains. The suspected positive colonies were detected with polymerase chain reaction amplifcation and sequencing of gltA, ftsZ, RNA polymerase beta subunit (rpoB) and ribC genes. Among 101 small mammals, 39 were positive for Bartonella, with the infection rate of 38.61%. The infection rate in diferent tissues (spleens and brains) (χ2 = 0.112, P = 0.738) and gender (χ2 = 1.927, P = 0.165) of small mammals did not have statistical diference, but that in diferent habitats had statistical diference (χ2 = 10.361, P = 0.016). Through genetic evolution analysis, 40 Bartonella strains were identifed (two diferent Bartonella species were detected in one small mammal), including B. grahamii (30), B. jaculi (3), B. krasnovii (3) and Candidatus B. gerbillinarum (4), which showed rodent-specifc characteristics. B. grahamii was the dominant epidemic strain (accounted for 75.0%). Furthermore, phylogenetic analysis showed that B. grahamii in the Qaidam Basin, might be close to the strains isolated from Japan and China. -
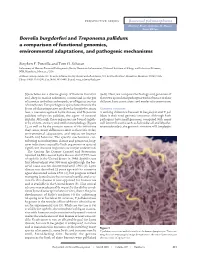
Borrelia Burgdorferi and Treponema Pallidum: a Comparison of Functional Genomics, Environmental Adaptations, and Pathogenic Mechanisms
PERSPECTIVE SERIES Bacterial polymorphisms Martin J. Blaser and James M. Musser, Series Editors Borrelia burgdorferi and Treponema pallidum: a comparison of functional genomics, environmental adaptations, and pathogenic mechanisms Stephen F. Porcella and Tom G. Schwan Laboratory of Human Bacterial Pathogenesis, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, NIH, Hamilton, Montana, USA Address correspondence to: Tom G. Schwan, Rocky Mountain Laboratories, 903 South 4th Street, Hamilton, Montana 59840, USA. Phone: (406) 363-9250; Fax: (406) 363-9445; E-mail: [email protected]. Spirochetes are a diverse group of bacteria found in (6–8). Here, we compare the biology and genomes of soil, deep in marine sediments, commensal in the gut these two spirochetal pathogens with reference to their of termites and other arthropods, or obligate parasites different host associations and modes of transmission. of vertebrates. Two pathogenic spirochetes that are the focus of this perspective are Borrelia burgdorferi sensu Genomic structure lato, a causative agent of Lyme disease, and Treponema A striking difference between B. burgdorferi and T. pal- pallidum subspecies pallidum, the agent of venereal lidum is their total genomic structure. Although both syphilis. Although these organisms are bound togeth- pathogens have small genomes, compared with many er by ancient ancestry and similar morphology (Figure well known bacteria such as Escherichia coli and Mycobac- 1), as well as by the protean nature of the infections terium tuberculosis, the genomic structure of B. burgdorferi they cause, many differences exist in their life cycles, environmental adaptations, and impact on human health and behavior. The specific mechanisms con- tributing to multisystem disease and persistent, long- term infections caused by both organisms in spite of significant immune responses are not yet understood. -

Novel Small Rnas Expressed by Bartonella Bacilliformis Under Multiple Conditions 2 Reveal Potential Mechanisms for Persistence in the Sand Fly Vector and Human 3 Host
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.04.235903; this version posted August 4, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Novel small RNAs expressed by Bartonella bacilliformis under multiple conditions 2 reveal potential mechanisms for persistence in the sand fly vector and human 3 host 4 5 6 Shaun Wachter1, Linda D. Hicks1, Rahul Raghavan2 and Michael F. Minnick1* 7 8 9 10 1 Program in Cellular, Molecular & Microbial Biology, Division of Biological Sciences, 11 University of Montana, Missoula, Montana, United States of America 12 2 Department of Biology and Center for Life in Extreme Environments, Portland State 13 University, Portland, Oregon, United States of America 14 15 16 17 * Corresponding author 18 E-mail: [email protected] (MFM) 19 20 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.04.235903; this version posted August 4, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 21 Abstract 22 Bartonella bacilliformis, the etiological agent of Carrión’s disease, is a Gram-negative, 23 facultative intracellular alphaproteobacterium. Carrión’s disease is an emerging but neglected 24 tropical illness endemic to Peru, Colombia, and Ecuador. B. bacilliformis is spread between 25 humans through the bite of female phlebotomine sand flies. -

09 Piqueras.Qxp
PERSPECTIVES INTERNATIONAL MICROBIOLOGY (2007) 10:217-226 DOI: 10.2436/20.1501.01.30 ISSN: 1139-6709 www.im.microbios.org Microbiology: a dangerous profession? Mercè Piqueras President, Catalan Association for Science Communication (ACCC), Barcelona, Spain The history of science contains many cases of researchers eases in Minorca from the year 1744 to 1749 to which is pre- who have died because of their professional activity. In the fixed, a short account of the climate, productions, inhabi- field of microbiology, some have died or have come close to tants, and endemical distempers of that island (T. Cadell, D. death from infection by agents that were the subject of their Wilson and G. Nicol, London, 1751), which he dedicated to research (Table 1). Infections that had a lethal outcome were the Society of Surgeons of His Majesty’s Royal Navy. usually accidental. Sometimes, however, researchers inocu- Minorcan historian of science Josep M. Vidal Hernández has lated themselves with the pathogen or did not take preventive described and carefully analyzed Cleghorn’s work in measures against the potential pathogen because they wanted Minorca and his report [43]. According to Vidal, what to prove their hypotheses—or disprove someone else’s— Cleghorn describes is “tertian” fever, which was the name regarding the origin of the infection. Here is an overview of given at the time to fever caused by malaria parasites with a several episodes in the history of microbiology since the mid periodicity of 48 hours. In fact, Cleghorn used quinine to nineteenth century involving researchers or workers in fields treat tertian fever (i.e, malaria), which was not eradicated related to microbiology who have become infected. -

Bartonella Henselae • Fleas and Black-Legged Ticks (Also Called Deer Ticks) of the Genus Ixodes May Serve As Vectors, but This Has Not Disease Agent: Been Proven
APPENDIX 2 Bartonella henselae • Fleas and black-legged ticks (also called deer ticks) of the genus Ixodes may serve as vectors, but this has not Disease Agent: been proven. • Bartonella henselae Blood Phase: Disease Agent Characteristics: • Agent found in endothelial cells and associated with RBCs in symptomatic cases • Gram-negative bacillus or coccobacillus, aerobic, • Occult bacteremia sometimes occurs in the absence nonmotile, nonspore-forming, facultatively intracel- of specific antibodies. lular bacterium • Order: Rhizobiales; Family: Bartonellaceae Survival/Persistence in Blood Products: • Size: 0.3-0.6 ¥ 0.3-1.0 mm • Nucleic acid: Approximately 1900 kb of DNA • A spiking study suggests that B. henselae added to RBCs can be recovered on solid media through 35 Disease Name: days of storage at 4°C. • Cat scratch disease • Cat scratch fever Transmission by Blood Transfusion: • Bacillary angiomatosis • Theoretical • Bacillary peliosis Cases/Frequency in Population: Priority Level: • 22,000 cases per year estimated in the US • Scientific/Epidemiologic evidence regarding blood • 2-6% in US blood donors safety: Theoretical • Cumulative seroprevalence of 7.1% to B. henselae and • Public perception and/or regulatory concern regard- B. quintana in US veterinary professionals ing blood safety: Absent • Public concern regarding disease agent: Very low Incubation Period: Background: • 3-10 days to appearance of papule at inoculation site; regional adenopathy may follow after a few weeks • In 1909,ALBartondescribed organisms that adhered to RBCs. Likelihood of Clinical Disease: • The name Bartonella bacilliformis was used for the • Relatively benign and self-limiting, lasting 6-12 weeks only member of the group identified before 1993. in the absence of antibiotic therapy • Several other species of Bartonella are known to infect humans, but at present, B. -

Phenotypic and Molecular Analysis of Desensitization to Levamisole in Male and Female Adult Brugia Malayi
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2019 Phenotypic and molecular analysis of desensitization to levamisole in male and female adult Brugia malayi Mengisteab T. Wolday Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Toxicology Commons Recommended Citation Wolday, Mengisteab T., "Phenotypic and molecular analysis of desensitization to levamisole in male and female adult Brugia malayi" (2019). Graduate Theses and Dissertations. 17809. https://lib.dr.iastate.edu/etd/17809 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Phenotypic and molecular analysis of desensitization to levamisole in male and female adult Brugia malayi by Mengisteab Wolday A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Toxicology Program of Study Committee: Richard J. Martin, Major Professor Alan P. Robertson Aileen F. Keating The student author, whose presentation of the scholarship herein was approved by the program of study committee, is solely responsible for the content of this thesis. The Graduate College will ensure this thesis is globally accessible and will not permit alterations after a degree is conferred. Iowa State University Ames, Iowa 2019 Copyright © Mengisteab Wolday, 2019. All rights reserved. ii DEDICATION This Thesis research is dedicated to my loving and caring parents Tesfaldet Wolday and Mizan Teckleab, and my siblings Mussie, Selam, Daniel, Yonas, Alek and Abraham, and my son Yosias. -

The Relapsing Fever Agent Borrelia Hermsii Has Multiple
Copyright Q 1992 by the Genetics Societyof America The Relapsing Fever AgentBorrelia hermsii Has Multiple Copiesof Its Chromosome and Linear Plasmids Todd Kitten and Alan G. Barbour Departments of Microbiology and Medicine, The Universityof Texas Health Science Center, Sun Antonio, Texas 78284 Manuscript received March 17, 1992 Accepted for publicationJune 25, 1992 ABSTRACT Borrelia hermsii, a spirochete which causes relapsing fever in humans and other mammals, eludes the immune responseby antigenic variationof the “Vmp” proteins.This occurs by replacement of an expressed vrnp gene with a copy of a silent vrnp gene. Silent and expressedvrnp genes are located on separate linearplasmids. To further characterize vrnp recombination, copy numbers were determined for two linear plasmids and for the l-megabase chromosome by comparing hybridization of probes to native DNA with hybridization to recombinant plasmids containing borrelial DNA. Plasmid copy numbers were also estimated by ethidium bromide fluorescence. Total cellular DNA content was determined by spectrophotometry. For borreliasgrown in mice, copy numbers and 95% confidence intervals were 14 (12-17)for an expression plasmid,8 (7-9) for a silent plasmid, and 16 (13-18) for the chromosome. Borrelias grown in broth mediumhad one-fourth to one-half this number of plasmids and chromosomes. Staining of cells with4’,6-diamidino-2-phenylindole revealed DNA to be distributed throughout most of the spirochete’s length. These findings indicate that borrelias organize their total cellular DNA into several complete genomes and thatcells undergoing serotype switches do one or more of the following: (1) coexpress Vmps from switched and unswitched expression plasmids for at least three to five generations, (2)suppress transcription from some expressionplasmid copies, or (3) partition expression plasmids nonrandomly.The lower copy number ofthe silentplasmid indicates that nonreciprocal Vmp gene recombination may result from loss of recombinant silent ” plasmids by segregation.