The Dynamics of Flower Development in Castanea Sativa Mill
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHESTNUT (CASTANEA Spp.) CULTIVAR EVALUATION for COMMERCIAL CHESTNUT PRODUCTION
CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE By Ana Maria Metaxas Approved: James Hill Craddock Jennifer Boyd Professor of Biological Sciences Assistant Professor of Biological and Environmental Sciences (Director of Thesis) (Committee Member) Gregory Reighard Jeffery Elwell Professor of Horticulture Dean, College of Arts and Sciences (Committee Member) A. Jerald Ainsworth Dean of the Graduate School CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE by Ana Maria Metaxas A Thesis Submitted to the Faculty of the University of Tennessee at Chattanooga in Partial Fulfillment of the Requirements for the Degree of Master of Science in Environmental Science May 2013 ii ABSTRACT Chestnut cultivars were evaluated for their commercial applicability under the environmental conditions in Hamilton County, TN at 35°13ꞌ 45ꞌꞌ N 85° 00ꞌ 03.97ꞌꞌ W elevation 230 meters. In 2003 and 2004, 534 trees were planted, representing 64 different cultivars, varieties, and species. Twenty trees from each of 20 different cultivars were planted as five-tree plots in a randomized complete block design in four blocks of 100 trees each, amounting to 400 trees. The remaining 44 chestnut cultivars, varieties, and species served as a germplasm collection. These were planted in guard rows surrounding the four blocks in completely randomized, single-tree plots. In the analysis, we investigated our collection predominantly with the aim to: 1) discover the degree of acclimation of grower- recommended cultivars to southeastern Tennessee climatic conditions and 2) ascertain the cultivars’ ability to survive in the area with Cryphonectria parasitica and other chestnut diseases and pests present. -

Landscaping and Gardening with Native Plants in Georgia's Coastal
Dry woodlands with scattered canopy trees WHAT ARE NATIVE PLANTS? often support a profusion of fall blooming & Native Plants, species that grew naturally in this wildflowers such as sunflowers, goldenrods, region prior to the colonial era, are uniquely G with and blazing stars. The upland forest with adapted to local conditions. They are suited to both the highest soil moisture occurs on slopes the physical and biological conditions of their native Native Plants where resistance to fire allows a variety of areas. trees such as American beech, white oak, BENEFITS OF NATIVES Georgia’s spruce pine, and southern magnolia to Promote biodiversity grow, as well as shrubs like Carolina Lower landscape & garden maintenance once Coastal buckthorn, needle palm, red buckeye, horse established by reducing the need for fertilizer, sugar, fringe tree, and native azaleas. pesticides and watering. Where soils have higher moisture and Foster appreciation of our natural mineral levels, the ground layer is rich and heritage and the beauty of our native reminiscent of the Southern Appalachians landscape with spring flowering plants like bloodroot, Native flora provide food and shelter tailored May apple, trout lily, doll’s eyes and to wildlife Solomon’s seal. By maintaining natural habitats and mending Coastal Plain of G LOWLANDS AND WETLANDS are areas those that are fragmented, you can make a Climate Zone: 8B Eco-regions 65 - 75 where the soil is inundated or saturated for a difference whether working at the landscape portion of each year, and include both riverine level or planting in containers. THE COASTAL PLAIN OF GEORGIA in this BASICS FOR USING NATIVES and isolated wetlands. -

Quercus Robur (Fagaceae)
Quercus robur (Fagaceae) The map description EEBIO area The integrated map shows the distribution and changes in the areal’s boundaries of pedunculate oak (Quercus robur). Q. robur is the dominant forest- formative species in the belt of broadleaf and mixed 4 needleleaf-broadleaf forests in the plains of the European part of the former USSR (Sokolov et al. 1977). In the northern part of its areal Q. robur grows in river valleys. In the central part, it forms mixed forests with Picea abies; closer to the south – a belt of broadleaf forests where Q. robur dominates. At the areal’s south boundary it forms small (marginal) forests in ravines and flood-plains (Atlas of Areals and Resources… , 1976). Q. robur belongs to the thermophilic species. The low temperature bound of possible occurrence of oak forests is marked by an average annual of 2?C l (http://www.forest.ru – in Russian). Therefore, l l l l l l hypotretically, oak areal boundaries will shift along l ll l l l l l l l with the changes in the average annual temperature. l l l l l l l l l l l For Yearly map of averaged mean annual air l l l l l l l l l l l l l l l l l l lll temperature (Afonin A., Lipiyaynen K., Tsepelev V., l l l l l l l ll ll l l l 2005) see http://www.agroatlas.spb.ru Climate. l l l l l l l l l l l l l l l l Oak forests are of great importance for the water l l l l l ll l lll l l l l l l l l l l l l l l l l l l l regime and soil structure, especially on the steep l l l l l l l l l l l l l l l l l l l l l l l l l l l l l slopes of river valleys and in forest-poor areas. -

5 Fagaceae Trees
CHAPTER 5 5 Fagaceae Trees Antoine Kremerl, Manuela Casasoli2,Teresa ~arreneche~,Catherine Bod6n2s1, Paul Sisco4,Thomas ~ubisiak~,Marta Scalfi6, Stefano Leonardi6,Erica ~akker~,Joukje ~uiteveld', Jeanne ~omero-Seversong, Kathiravetpillai Arumuganathanlo, Jeremy ~eror~',Caroline scotti-~aintagne", Guy Roussell, Maria Evangelista Bertocchil, Christian kxerl2,Ilga porth13, Fred ~ebard'~,Catherine clark15, John carlson16, Christophe Plomionl, Hans-Peter Koelewijn8, and Fiorella villani17 UMR Biodiversiti Genes & Communautis, INRA, 69 Route d'Arcachon, 33612 Cestas, France, e-mail: [email protected] Dipartimento di Biologia Vegetale, Universita "La Sapienza", Piazza A. Moro 5,00185 Rome, Italy Unite de Recherche sur les Especes Fruitikres et la Vigne, INRA, 71 Avenue Edouard Bourlaux, 33883 Villenave d'Ornon, France The American Chestnut Foundation, One Oak Plaza, Suite 308 Asheville, NC 28801, USA Southern Institute of Forest Genetics, USDA-Forest Service, 23332 Highway 67, Saucier, MS 39574-9344, USA Dipartimento di Scienze Ambientali, Universitk di Parma, Parco Area delle Scienze 1lIA, 43100 Parma, Italy Department of Ecology and Evolution, University of Chicago, 5801 South Ellis Avenue, Chicago, IL 60637, USA Alterra Wageningen UR, Centre for Ecosystem Studies, P.O. Box 47,6700 AA Wageningen, The Netherlands Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46556, USA lo Flow Cytometry and Imaging Core Laboratory, Benaroya Research Institute at Virginia Mason, 1201 Ninth Avenue, Seattle, WA 98101, -

The Quantitative Importance of Stemflow: an Evaluation of Past Research and Results from a Study in Lodgepole Pine (Pinus Contorta Var
THE QUANTITATIVE IMPORTANCE OF STEMFLOW: AN EVALUATION OF PAST RESEARCH AND RESULTS FROM A STUDY IN LODGEPOLE PINE (PINUS CONTORTA VAR. LATIFOLIA) STANDS IN SOUTHERN BRITISH COLUMBIA by Adam Jon McKee B.A. Thompson Rivers University, 2008 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE (ENVIRONMENTAL SCIENCE) Thesis examining committee: Darryl Carlyle-Moses (Ph.D.), Thesis Supervisor, Assistant Professor, Dept. of Geography, Thompson Rivers University Karl Larsen (Ph.D.), Committee Member, Associate Professor, Dept. of Natural Resource Sciences, Thompson Rivers University Rita Winkler (Ph.D., R.P.F.), Committee Member, Adjunct Professor, Dept. of Natural Resource Sciences and Research Hydrologist, BC Ministry of Forests and Range Delphis F. Levia (Ph.D.), External Examiner, Associate Professor, Depts. of Geography & Plant and Soil Science, University of Delaware Spring Convocation 2011 Thompson Rivers University © Adam Jon McKee, 2010 Thesis Supervisory Committee ________________________ Dr. Darryl Carlyle-Moses, Supervisor ________________________ Dr. Karl Larsen, Committee Member ________________________ Dr. Rita Winkler, Committee Member This thesis by Adam Jon McKee was defended successfully in an oral examination on December 9, 2010 by a committee comprising: ________________________ Dr. Delphis F. Levia, External Examiner ________________________ Dr. Darryl Carlyle-Moses, Supervisor ________________________ Dr. Karl Larsen, Committee Member ________________________ Dr. Rita Winkler, Committee Member ii ________________________ Dr. Lauchlan Fraser, Chair/Coordinator of Graduate Program Committee ________________________ Dr. Tom Dickinson, Dean of Science ________________________ Dr. Peter Tsigaris, Chair of the Examining Committee This thesis is accepted in its present form by the Office of the Associate Vice President, Research and Graduate Studies as satisfying the thesis requirements for the degree Master of Science, Environmental Science. -

Diversity of Wisconsin Rosids
Diversity of Wisconsin Rosids . oaks, birches, evening primroses . a major group of the woody plants (trees/shrubs) present at your sites The Wind Pollinated Trees • Alternate leaved tree families • Wind pollinated with ament/catkin inflorescences • Nut fruits = 1 seeded, unilocular, indehiscent (example - acorn) *Juglandaceae - walnut family Well known family containing walnuts, hickories, and pecans Only 7 genera and ca. 50 species worldwide, with only 2 genera and 4 species in Wisconsin Carya ovata Juglans cinera shagbark hickory Butternut, white walnut *Juglandaceae - walnut family Leaves pinnately compound, alternate (walnuts have smallest leaflets at tip) Leaves often aromatic from resinous peltate glands; allelopathic to other plants Carya ovata Juglans cinera shagbark hickory Butternut, white walnut *Juglandaceae - walnut family The chambered pith in center of young stems in Juglans (walnuts) separates it from un- chambered pith in Carya (hickories) Juglans regia English walnut *Juglandaceae - walnut family Trees are monoecious Wind pollinated Female flower Male inflorescence Juglans nigra Black walnut *Juglandaceae - walnut family Male flowers apetalous and arranged in pendulous (drooping) catkins or aments on last year’s woody growth Calyx small; each flower with a bract CA 3-6 CO 0 A 3-∞ G 0 Juglans cinera Butternut, white walnut *Juglandaceae - walnut family Female flowers apetalous and terminal Calyx cup-shaped and persistant; 2 stigma feathery; bracted CA (4) CO 0 A 0 G (2-3) Juglans cinera Juglans nigra Butternut, white -

ISTA List of Stabilized Plant Names 7Th Edition
ISTA List of Stabilized Plant Names th 7 Edition ISTA Nomenclature Committee Chair: Dr. M. Schori Published by All rights reserved. No part of this publication may be The Internation Seed Testing Association (ISTA) reproduced, stored in any retrieval system or transmitted Zürichstr. 50, CH-8303 Bassersdorf, Switzerland in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior ©2020 International Seed Testing Association (ISTA) permission in writing from ISTA. ISBN 978-3-906549-77-4 ISTA List of Stabilized Plant Names 1st Edition 1966 ISTA Nomenclature Committee Chair: Prof P. A. Linehan 2nd Edition 1983 ISTA Nomenclature Committee Chair: Dr. H. Pirson 3rd Edition 1988 ISTA Nomenclature Committee Chair: Dr. W. A. Brandenburg 4th Edition 2001 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 5th Edition 2007 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 6th Edition 2013 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 7th Edition 2019 ISTA Nomenclature Committee Chair: Dr. M. Schori 2 7th Edition ISTA List of Stabilized Plant Names Content Preface .......................................................................................................................................................... 4 Acknowledgements ....................................................................................................................................... 6 Symbols and Abbreviations .......................................................................................................................... -
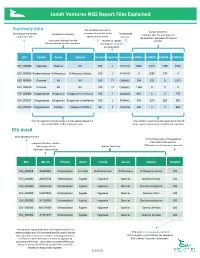
NGS Report Explained
Jonah Ventures NGS Report Files Explained Summary data The % of base pairs in the Sample identifiers Exact Sequence Variant sequence that match to the The detected Exact Sequence Variant Consensus Taxonomy The detected Numbers after the decimal point unique identifier species in the library. sequence. represent lab replicates of the same Taxonomic ranking from the Number of species sample. library matched to each sequence. matching the sequence at a given level. ESV Family Genus Species %match # Species Sequence S10001.1 S10002.1 S10003.1 S10003.2 ESV_000080 Fagaceae Quercus NA 100 7 AAAAAG… 3066 1340 5780 3462 ESV_000818 Orobanchaceae Orthocarpus Orthocarpus luteus 100 1 AAAAAG… 0 2582 249 0 ESV_000006 Poaceae NA NA 100 77 GAAAAG… 584 101 0 1039 ESV_000050 Poaceae NA NA 100 12 GAAAAG… 1328 0 0 0 ESV_000308 Polygonaceae Polygonum Polygonum humifusum 100 2 AAAAAG… 902 0 0 278 ESV_000027 Polygonaceae Eriogonum Eriogonum umbellatum 100 1 ATAAAG… 341 124 282 381 ESV_000520 Polygonaceae Fallopia Fallopia multiflora 99 1 AAAAAG… 126 0 0 994 “NA” at a taxonomic ranks means multiple species present in The number in each cell is the absolute number of the sample differ in that taxonomic rank times a given sequence was read by the sequencer. ESV detail Exact Sequence Variant The % of base pairs in the sequence that match to the species. Index identification number. that match to the species. Each sample has an Species Taxonomy 100% indicates all base pairs matched individual index number. ESV GB_ID Phylum Order Family Genus Species %match ESV_000818 -

Aspects of Ecology and Conservation of the Pygmy Loris Nycticebus Pygmaeus in Vietnam
Aus dem Institut für Zoologie, Fischkrankheiten und Fischereibiologie der Tierärztlichen Fakultät der Ludwig-Maximilians-Universität München Angefertigt am Endangered Primate Rescue Center Cuc Phuong National Park Vietnam Aspects of Ecology and Conservation of the Pygmy Loris Nycticebus pygmaeus in Vietnam Inaugural-Dissertation zur Erlangung der tiermedizinischen Doktorwürde der Tierärztlichen Fakultät der Ludwig-Maximilians Universität München vorgelegt von Ulrike Streicher aus Bamberg München, Oktober 2004 Dem Andenken meines Vaters Preface The first pygmy lorises came to the Endangered Primate Rescue Center in 1995 and were not much more than the hobby of the first animal keeper, Manuela Klöden. They were at that time, even by Vietnamese scientists or foreign primate experts, considered not very important. They were abundant in the trade and there was little concern about their wild status. It has often been the fate of animals that are considered common not to be considered worth detailed studies. But working with confiscated pygmy lorises we discovered a number of interesting facts about them. They seasonally changed the pelage colour, they showed regular weight variations, and they did not eat in certain times of the year. And I met people interested in lorises and told them, what I had observed and realized these facts were not known. So I started to collect data more or less to proof what we had observed at the centre. Due to the daily veterinary tasks data collection was rather randomly and unfocussed. But the more we got to know about the pygmy lorises, the more interesting it became. The answer to one question immediately generated a number of consecutive questions. -

Chestnuts in Appalachian Culture Part II Chestnuts in Appalachian Culture Part II a Perfect Wildlife Food Lost in Time, But
the September 2010 | Issue 2 Vol.24 27th Annual Meeting October 15-17 Registration Information Inside Chestnuts in Appalachian Culture Part II A Perfect Wildlife Food Lost in Time, But Not Forgotten Simple Strategies for Controlling a Common Pest MeadowviewMeadowview DedicationDedication a Success!S ! 27th REGISTER ONLINE AT WWW.ACF.ORG REGISTRATIONANNUAL MEETING OR CALL (828) 281-0047 TO REGISTER BY PHONE THE AMERICAN CHESTNUT FOUNDATION Option 1: Full Registration PAYMENT TACF Member $75 Name of Attendee(s) Non-Member $115 (includes a one-year membership) Address Full Registration for one person City (does not include lodging) State Includes: Zip Code Phone number t Friday Night Welcome Reception t Saturday Night Dinner & Awards Program Email t Access to all Workshops Form of Payment t All Meals Check (payable to TACF) Credit Card Option 2: Day Passes for Workshops Only (Registration fee does not include lodging Total amount due $ or meals) Credit Card Billing Information SATURDAY Credit Card (circle one): Visa Mastercard Regular Members $40 Card Number __ __ __ __-__ __ __ __-__ __ __ __-__ __ __ __ Student Members $40 Regular Non-Member $80 (includes a one-year membership) Expiration Date Student Non-Member $55 (includes a one-year membership) Name on Card (print) SUNDAY Address Regular Members $25 City Student Members $25 State Zip Code Regular Non-Member $65 (includes a one-year membership) Student Non-Member $40 (includes a one-year membership) Phone number All attendees MUST pre-register for the Annual Meeting. Signature TACF needs to register all of our attendees with NCTC’s security office prior to the meeting, and no on-site Return form and payment to: registration will be available. -

Two-Step Contractions of Inverted Repeat Region and Psai Gene Duplication from the Plastome of Croton Tiglium (Euphorbiaceae)
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 23 November 2018 doi:10.20944/preprints201807.0458.v2 Two-Step Contractions of Inverted Repeat Region and psaI Gene Duplication from the Plastome of Croton tiglium (Euphorbiaceae) Sangjin Jo and Ki-Joong Kim School of Life Sciences, Korea University, Seoul 02841, Korea Corresponding author Ki-Joong Kim Anam-ro, Seoul, 02841, Korea Email address: [email protected] Abstract Croton L. (Euphorbiaceae) is a very specious genus and consists of about 1,250 species, mainly distributed in the New World. The first complete plastome sequence from the genus, Croton tiglium, is reported in this study (NCBI acc. no. MH394334). The plastome is 150,021 bp in length. The lengths of LSC and SSC are 111,654 bp and 18,167 bp, respectively. However, the length of the IR region is only 10,100 bp and includes only four rrn and four trn genes, and a small part of the ycf1 gene. We propose two-step IR contractions to explain this unique IR region of the C. tiglium plastome. First, the IR contracted from rps19-rpl2 to ycf2-trnL-CAA on the LSC/IRb boundary. Second, the IR contracted from ycf2-trnL-CAA to rrn16-trnV-GAC on the LSC/IRa boundary. In addition, duplicated copies of psaI genes were discovered in the C. tiglium plastome. Both copies were located side by side between accD and ycf4 genes, but one copy was pseudogenized because of a five-basepair (TAGCT) insertion in the middle of the gene and following frameshift mutation. The plastome contains 112 genes, of which 78 are protein-coding genes, 30 are tRNA genes, and four are rRNA genes. -

Viburnum L. Viburnum
VWYZ genera Layout 1/31/08 1:11 PM Page 1154 V Ericaceae—Heath family Vaccinium L. blueberry, cranberry Jason J. Griffin and Frank A. Blazich Dr. Griffin is assistant professor at Kansas State University’s Department of Horticulture, Forestry, and Recreation Resources, Manhatten, Kansas; Dr. Blazich is alumni distinguished graduate professor of plant propagation and tissue culture at North Carolina State University’s Department of Horticultural Science, Raleigh, North Carolina Occurrence, growth habit, and uses. There are about Although cranberry has been introduced successfully 150 to 450 species (the number varies by authority) of into cultivation in British Columbia, Washington, and deciduous or evergreen shrubs (rarely trees or vines) in Oregon, Wisconsin and Massachusetts remain the largest Vaccinium (Huxley 1992b; LHBH 1976; Vander Kloet producers; the crops for 2000 were estimated at 2.95 and 1988). The majority of species are native to North and South 1.64 million barrels, respectively (NASS 2001). America and eastern Asia (LHBH 1976; Vander Kloet Evergreen huckleberry grows along the Pacific Coast 1988). Some of the more commonly cultivated North and is valued for its attractive foliage, which is often used in American species are listed in table 1. Like other members flower arrangements (Everett 1981). Species of Vaccinium of the Ericaceae, Vaccinium species require an acidic (pH also are prized as landscape plants. Lowbush forms are used 4.0 to 5.2) soil that is moist, well drained, and high in organ- to form attractive ground covers or shrubs. Two cultivars of ic matter (3 to 15%). Symptoms of mineral nutrient defi- creeping blueberry (V.