Comparative Study of the Composition of Sweat from Eccrine and Apocrine Sweat Glands During Exercise and in Heat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Distribution of Sweat Glands Over the Human Body Has So Far Been In
NOTES ON THE VERTICAL DISTRIBUTION OF THE HUMAN SWEAT GLANDS SHUNZO TAKAGI AND KO TOBARU* Institute of Physiology, School of Medicine, University of Nagoya•õ The distribution of sweat glands over the human body has so far been in- vestigated in the dimension of area, and we have no general idea how deep they are distributed in the skin. In 1943, Kuno and his collaborators (3) expressed the opinion that chloride would be accumulated in the skin during the activity of sweat glands. The truth of this assumption has been confirmed with more certainty by Yoshimura and Chihaya (unpublished), who measured the chloride content in the skin tissue by means of Ag-AgCl electrodes. The chloride may presumably be accumulated in the immediate neighbourhood of the glomeruli of sweat glands, or more diffusely in the layers of skin tissues where the glomeruli are situated. For consideration of the amount of the accumulated chloride, the total volume of these skin layers, which can be estimated by the vertical distribution of the sweat-gland glomeruli, seems to be useful. The fol- lowing investigation was therefore performed. MATERIALS AND METHOD Skin samples of 33 regions of the body, as specified in table 1, were taken from the corpse of a Japanese male of 30 years old, who died an accidental death. The samples were fixed in 10 per cent formalin, embedded in celloidin and cut into sections 15 micra thick. The sections were stained with Delafield's hematoxylin and eosin. Observations of sweat glands were made with 2-3 pieces of the skin about 100 sq. -

Long-Lasting Muscle Thinning Induced by Infrared Irradiation Specialized with Wavelengths and Contact Cooling: a Preliminary Report
Long-Lasting Muscle Thinning Induced by Infrared Irradiation Specialized With Wavelengths and Contact Cooling: A Preliminary Report Yohei Tanaka, MD, Kiyoshi Matsuo, MD, PhD, and Shunsuke Yuzuriha, MD, PhD Department of Plastic and Reconstructive Surgery, Shinshu University School of Medicine, Matsumoto, Nagano 390-8621, Japan Correspondence: [email protected] Published May 28, 2010 Objective: Infrared (IR) irradiation specialized with wavelengths and contact cooling increases the amount of water in the dermis to protect the subcutaneous tissues against IR damage; thus, it is applied to smooth forehead wrinkles. However, this treatment consistently induces brow ptosis. Therefore, we investigated whether IR irradiation induces muscle thinning. Methods: Rat central back tissues were irradiated with the specialized IR device. Histological evaluation was performed on sagittal slices that included skin, panniculus carnosus, and deep muscles. Results: Significant reductions in panniculus carnosus thickness were observed between controls and irradiated tissues at postirradiation day 30 (P30), P60, P90, and P180; however, no reduction was observed in nonirradiated controls from days 0 to 180. No significant changes were observed in the trunk muscle over time. From day 0, dermal thickness was significantly reduced at P90 and P180; however, no difference was observed between P180 and nonirradiated controls at day 180. DNA degradation consistent with apoptosis was detected in the panniculus carnosus at P7 and P30. Conclusions: We found that IR irradiation induced long-lasting superficial muscle thinning, probably by a kind of apoptosis. The panniculus carnosus is equivalent to the superficial facial muscles of humans; thus, the changes observed here reflected those in the frontalis muscle that resulted in brow ptosis. -

A Spectral BSSRDF for Shading Human Skin
Eurographics Symposium on Rendering (2006) Tomas Akenine-Möller and Wolfgang Heidrich (Editors) A Spectral BSSRDF for Shading Human Skin Craig Donner and Henrik Wann Jensen† Universtiy of California at San Diego, La Jolla, CA, USA Abstract We present a novel spectral shading model for human skin. Our model accounts for both subsurface and surface scattering, and uses only four parameters to simulate the interaction of light with human skin. The four parameters control the amount of oil, melanin and hemoglobin in the skin, which makes it possible to match specific skin types. Using these parameters we generate custom wavelength dependent diffusion profiles for a two-layer skin model that account for subsurface scattering within the skin. These diffusion profiles are computed using convolved diffusion multipoles, enabling an accurate and rapid simulation of the subsurface scattering of light within skin. We combine the subsurface scattering simulation with a Torrance-Sparrow BRDF model to simulate the interaction of light with an oily layer at the surface of the skin. Our results demonstrate that this four parameter model makes it possible to simulate the range of natural appearance of human skin including African, Asian, and Caucasian skin types. Categories and Subject Descriptors (according to ACM CCS): I.3.7 [Computer Graphics]: Color, shading, shadowing, and texture 1 Introduction Debevec et al. [DHT∗00] measured the reflectance field of human faces, allowing for rendering of skin under varying Simulating the appearance of human skin is a challenging illumination conditions with excellent results. Jensen and problem due to the complex structure of the skin. Further- Buhler [JB02], Hery [Her03] and Weyrich et al. -

Isolation and Growth of Adult Human Epidermal Keratinocytes in Cell Culture
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector CITATION CLASSIC 0022-202X/78/7102-0157$02.00/0 THE JOURNAL OF INVESTIGATIVE DERMATOLOGY, 71:157–162, 1978 Vol. 71, No. 2 Copyright & 1978 by The Williams & Wilkins Co. PrintedinU.S.A. Isolation and Growth of Adult Human Epidermal Keratinocytes in Cell Culture SU-CHIN LIU,PH.D., AND MARVIN KARASEK,PH.D. Humanepidermalkeratinocytesmaybeisolatedinhighyieldfrom 0.1 mm keratotome sections of adult skin by short-term trypsinrelease.Whenplatedonacollagen-coatedplasticsurfaceor on a collagen gel, keratinocytes attach with high efficiencies (470%) and form confluent, stratified cultures. Cell populations of predominantly basal cells produce proliferative primary cell cultures while populations of basal cells and malpighian cells result in nonproliferative primary cultures. Both nonproliferative and proliferative primary cultures may be subcultured on collagen gels following dispersion by trypsin and EDTA. Methotrexate strongly inhibits proliferative keratinocytes at low concentrations (0.1 mg/ml) but has no cytotoxic effect on non- proliferative cells. L-serine and dexamethasone increase the multiplication rate of both primary and subcultured human keratinocytes. The ability to isolate and to grow human epidermal keratinocytes from Preparation of Collagen Surfaces both normal and diseased human skin in sufficient quantities for Acid soluble collagen is extracted and purified from adult rabbit skin as described biochemical and genetic studies has been a long-range goal of many previously [10]. Three types of culture surfaces are prepared on 35-mm plastic investigators. Although keratinocytes may be obtained from postem- Petri dishes: (a) collagen-coated, (b) thin gel, and (c) 2-mm collagen gel. -

An Analysis of Benign Human Prostate Offers Insights Into the Mechanism
www.nature.com/scientificreports OPEN An analysis of benign human prostate ofers insights into the mechanism of apocrine secretion Received: 12 March 2018 Accepted: 22 February 2019 and the origin of prostasomes Published: xx xx xxxx Nigel J. Fullwood 1, Alan J. Lawlor2, Pierre L. Martin-Hirsch3, Shyam S. Matanhelia3 & Francis L. Martin 4 The structure and function of normal human prostate is still not fully understood. Herein, we concentrate on the diferent cell types present in normal prostate, describing some previously unreported types and provide evidence that prostasomes are primarily produced by apocrine secretion. Patients (n = 10) undergoing TURP were prospectively consented based on their having a low risk of harbouring CaP. Scanning electron microscopy and transmission electron microscopy was used to characterise cell types and modes of secretion. Zinc levels were determined using Inductively Coupled Plasma Mass Spectrometry. Although merocrine secretory cells were noted, the majority of secretory cells appear to be apocrine; for the frst time, we clearly show high-resolution images of the stages of aposome secretion in human prostate. We also report a previously undescribed type of epithelial cell and the frst ultrastructural image of wrapping cells in human prostate stroma. The zinc levels in the tissues examined were uniformly high and X-ray microanalysis detected zinc in merocrine cells but not in prostasomes. We conclude that a signifcant proportion of prostasomes, possibly the majority, are generated via apocrine secretion. This fnding provides an explanation as to why so many large proteins, without a signal peptide sequence, are present in the prostatic fuid. Tere are many complications associated with the prostate from middle age onwards, including benign prostatic hyperplasia (BPH) and prostate cancer (PCa). -

Periodic Acid-Schiff Positive Material Accumulating Within the Lumen of Eccrine Sweat Glands*
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector PERIODIC ACID-SCHIFF POSITIVE MATERIAL ACCUMULATING WITHIN THE LUMEN OF ECCRINE SWEAT GLANDS* GEORGE W. HAMBRICK, JR., M.D. The purpose of this paper is to record findings with regard to diastase-resistant, periodic acid-Schiff positive material in the lumen of eccrine ducts and glands of two individuals. This material has two possible sources in the normal eccrine sweat gland, namely, the cuticle lining of the eccrine duct and the cells of the secretory tubule. Holyoke and Lobits (3) studying 35 normal skin biopsies re- B pp HI D FIG. 1. A. Vertical section through skin of the control area showing dilatation of an eccrine sweat duct with eosinophilic material in the lumen. H. and E., X178. B. Same find- ings as in A above from an area treated daily with 3 per cent hexachloronaphthalene in acetone for one week, Xl7S. C. Section through dermal parts of eccrine duct containing a cast. H. and E., X355. D. Section through dermal eccrine duct containing periodic acid- Schiff positive material in the lumen, X660. *Fromthe Department of Dermatology (Donald M. Pillsbury, M.D., Director), School of Medicine, University of Pennsylvania, Philadelphia 4, Pennsylvania. This study was supported by U. S. Army grant DA-49-007-MD-154. Received for publication March 29, 1957. 213 214 THF JOURNAL OF INVESTIGATIVE DERMATOLOGY ported the presence of material in the lumen of the eccrine duct and gland; they classified the material as amorphous, cast, cellular or bacterial. -

Apocrine Sweat Retention in Man I
CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector APOCRINE SWEAT RETENTION IN MAN I. EXPERIMENTAL PRODUCTION OF ASYMPTOMATIC FORM*, I. HARRYJ. HTJRLEY, JR.,M.D.ANnWALTERB. SHELLEY, M.D., PH.D. Within recent years, the concept of poral closure and the effects of such an obstructive process on the eccrine sweat gland have been clearly defined (1, 2). The pathogenesis of the various sweat retention syndromes is now more fully understood. Moreover, the need for further study of the "blockage factor" in the diseases of the other glandular appendages has been emphasized. Much attention has been paid to the sebaceous gland in this respect (3). Follicular occlusion has been long regarded as one of the essential phases in the development of the acneiform dermatoses. In contrast, however, the possible role of ductal obstruction and consequent sweat retention in the diseases of the apocrine sweat gland has received little mention in the literature. Yet an examination of the clinical features of the apocrine disorders indicates that poral occlusion may be of considerable signifi- cance in their pathogenesis. This series of investigations was undertaken in an effort to define the effects of ductal closure on the human apocrine sweat gland. METHOD AND MATERIALS Seven healthy adult white males between the ages of 20 and 28 were selected for this study. Antiperspirants had been interdicted for at least one month. The axillae of these subjects were shaved and the apocrine sweat glands emptied by the local subcutaneous injection of 0.15 cc. 1:1000 epinephrine so- lution. -

Sweat Gland Myoepithelial Cell Differentiation
Journal of Cell Science 112, 1925-1936 (1999) 1925 Printed in Great Britain © The Company of Biologists Limited 1999 JCS4638 Human sweat gland myoepithelial cells express a unique set of cytokeratins and reveal the potential for alternative epithelial and mesenchymal differentiation states in culture Margarete Schön1,*, Jennifer Benwood1, Therese O’Connell-Willstaedt2 and James G. Rheinwald1,2,‡ 1Division of Dermatology/Department of Medicine, Brigham and Women’s Hospital, and 2Division of Cell Growth and Regulation, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115, USA *Present address: Department of Dermatology, Heinrich-Heine University, Moorenstrasse 5, 40225 Düsseldorf, Germany ‡Author for correspondence (e-mail: [email protected]) Accepted 9 April; published on WWW 26 May 1999 SUMMARY We have characterized precisely the cytokeratin expression myoepithelial cells, a constituent of secretory glands. pattern of sweat gland myoepithelial cells and have Immunostaining of skin sections revealed that only sweat identified conditions for propagating this cell type and gland myoepithelial cells expressed the same pattern of modulating its differentiation in culture. Rare, unstratified keratins and α-sma and lack of E-cadherin as the cell type epithelioid colonies were identified in cultures initiated we had cultured. Interestingly, our immunocytochemical from several specimens of full-thickness human skin. These analysis of ndk, a skin-derived cell line of uncertain cells divided rapidly in medium containing serum, identity, suggests that this line is of myoepithelial origin. epidermal growth factor (EGF), and hydrocortisone, and Earlier immunohistochemical studies by others had found maintained a closely packed, epithelioid morphology when myoepithelial cells to be K7-negative. -
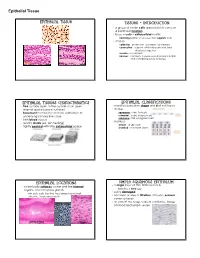
Epithelial Tissue
Epithelial Tissue Epithelial Tissue Tissues - Introduction · a group of similar cells specialized to carry on a particular function · tissue = cells + extracellular matrix nonliving portion of a tissue that supports cells · 4 types epithelial - protection, secretion, absorption connective - support soft body parts and bind structures together muscle - movement nervous - conducts impulses used to help control and coordinate body activities Epithelial Tissues Characteristics Epithelial Classifications · free surface open to the outside or an open · classified based on shape and # of cell layers internal space (apical surface) · shape · basement membrane anchors epithelium to squamous - thin, flat cells underlying connective tissue cuboidal - cube-shaped cells columnar - tall, elongated cells · lack blood vessels · number · readily divide (ex. skin healing) simple - single layer · tightly packed with little extracellular space stratified - 2 or more layers Epithelial Locations Simple Squamous Epithelium · a single layer of thin, flattened cells · cover body surfaces, cover and line internal organs, and compose glands looks like a fried egg · easily damaged skin cells, cells that line the stomach and small intestine, inside your mouth · common at sites of filtration, diffusion, osmosis; cover surfaces · air sacs of the lungs, walls of capillaries, linings cheek cells of blood and lymph vessels intestines skin Epithelial Tissue Simple Cuboidal Epithelium Simple Columnar Epithelium · single layer of cube-shaped cells · single layer of cells -

Table S1. Checklist for Documentation of Google Trends Research
Table S1. Checklist for Documentation of Google Trends research. Modified from Nuti et al. Section/Topic Checklist item Search Variables Access Date 11 February 2021 Time Period From January 2004 to 31 December 2019. Query Category All query categories were used Region Worldwide Countries with Low Search Excluded Volume Search Input Non-adjusted „Abrasion”, „Blister”, „Cafe au lait spots”, „Cellulite”, „Comedo”, „Dandruff”, „Eczema”, „Erythema”, „Eschar”, „Freckle”, „Hair loss”, „Hair loss pattern”, „Hiperpigmentation”, „Hives”, „Itch”, „Liver spots”, „Melanocytic nevus”, „Melasma”, „Nevus”, „Nodule”, „Papilloma”, „Papule”, „Perspiration”, „Petechia”, „Pustule”, „Scar”, „Skin fissure”, „Skin rash”, „Skin tag”, „Skin ulcer”, „Stretch marks”, „Telangiectasia”, „Vesicle”, „Wart”, „Xeroderma” Adjusted Topics: "Scar" + „Abrasion” / „Blister” / „Cafe au lait spots” / „Cellulite” / „Comedo” / „Dandruff” / „Eczema” / „Erythema” / „Eschar” / „Freckle” / „Hair loss” / „Hair loss pattern” / „Hiperpigmentation” / „Hives” / „Itch” / „Liver spots” / „Melanocytic nevus” / „Melasma” / „Nevus” / „Nodule” / „Papilloma” / „Papule” / „Perspiration” / „Petechia” / „Pustule” / „Skin fissure” / „Skin rash” / „Skin tag” / „Skin ulcer” / „Stretch marks” / „Telangiectasia” / „Vesicle” / „Wart” / „Xeroderma” Rationale for Search Strategy For Search Input The searched topics are related to dermatologic complaints. Because Google Trends enables to compare only five inputs at once we compared relative search volume of all topics with topic „Scar” (adjusted data). Therefore, -

There Are 25 Questions, Each Worth 3 Points and a Short Essay Worth 25 Points. DO YOUR OWN WORK !! Use Your Time Wisely 1. T
Mr. Holder Integumentary System – Unit 5 December 3, 2015 ARC TEST 1.0 DO NOT MARK OR WRITE ON THIS QUIZ !! There are 25 questions, each worth 3 points and a short essay worth 25 points. DO YOUR OWN WORK !! Use Your Time Wisely 1. The Integumentary System is divided into how many layers? a) 2 b) 3 c) 4 d) 6 2. What are the two major groups of membranes covering the human body? a) Epithelial & Mucus b) Cutaneous & Mucus c) Epithelial & Connective Tissue d) None of these 3. Which internal membrane provides protection for your joints? a) Serous b) Synovial c) Cutaneous d) Mucus 4. These membranes line internal cavities exposed to air & excrete a gooey substance. a) Serous b) Synovial c) Cutaneous d) Mucus 5. The Integumentary System protects the human body from … a) Friction b) Hot & Cold Temperature c) Bacteria d) All of These 6. Which stratum of the epidermis is full of keratin, cornified to prevent water loss? a) Basale b) Granulosum c) Corneum d) None of These 7. Which body system extends into the dermis to provide information to your brain? a) Cardiovascular b) Immune c) Integumentary d) Nervous 8. Subcutaneous tissue includes adipose tissue or fat. It is also known as the … a) Dermis b) Papillary Layer c) Hypodermis d) Reticular Layer 9. The dermis is divided into two layers. Which of these is the thickest? a) Papillary b) Reticular c) Basale d) Hypodermis 10. Which stratum of the epidermis is responsible for new cell production? a) Corneum b) Basale c) Granulosum d) Spinosum Page 1 Mr. -

The Anatomy of the Skin of the Chinese Tree Shrew Is Very Similar to That of Human Skin
ZOOLOGICAL RESEARCH The anatomy of the skin of the Chinese tree shrew is very similar to that of human skin DEAR EDITOR, murine model induced by imiquimod (Chuang et al., 2018) and inflammatory mouse model of Behçet's disease induced by The Chinese tree shrew (Tupaia belangeri chinensis) is a HSV-1 (Islam & Sohn, 2018), but the species disparity small mammal closely related to primates. It has a small body sometimes makes them difficult to extrapolate (van der Worp size, low maintenance cost, and a relatively short reproductive et al., 2010). Non-human primates (NHP), like rhesus cycle, all of which has made it the ideal model for the study of macaques, are genetically closer to humans and have a variety of human diseases. In this study, we compared the significant benefits in medical research (Buffalo et al., 2019; anatomy of the skin of the Chinese tree shrew with that of the Zhang et al., 2014). NHP has been used to explore responses rhesus macaque, mouse and human, with the intention of of Leishmania (Viannia) braziliensis cutaneous infection to N- providing the basic data required for the creation of skin methylglucamine antimoniate (Teva et al., 2005) and as disease models using this animal. Paraffin sections, SIVmac239-infected model for studying HIV infection (Zhang hematoxylin-eosin (H&E) staining, masson staining and et al., 2019), to name a few. The NHP model has been proved immunohistochemical techniques were used to examine the to be the best model for biomedical researches. However, dorsal skin structure of the Chinese tree shrew. The epidermis there are also some disadvantages of using NHP as the was shown to be composed of 1–2 layers of cells.