Xenon, Compressed SDS P-4677
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

First Xenon-Xenon Collisions in the Lhc
9th International Particle Accelerator Conference IPAC2018, Vancouver, BC, Canada JACoW Publishing ISBN: 978-3-95450-184-7 doi:10.18429/JACoW-IPAC2018-MOPMF039 FIRST XENON-XENON COLLISIONS IN THE LHC M. Schaumann∗, R. Alemany-Fernandez, P. Baudrenghien, T. Bohl, C. Bracco, R. Bruce, N. Fuster-Martinez, M. A. Jebramcik, J.M. Jowett, T. Mertens, D. Mirarchi, S. Redaelli, B. Salvachua, M. Solfaroli, H. Timko, J. Wenninger, CERN, Geneva, Switzerland Abstract EXECUTION OF THE RUN In 2017, the CERN accelerator complex once again demonstrated its flexibility by producing beams of a new A total of about 18 h of LHC time were taken for the Xe ion species, xenon, that were successfully injected into LHC. collision run. The schedule was designed to include set-up, On 12 October, collisions of fully stripped xenon nuclei first injection, validation and physics data taking. A further 12 h were devoted to experiments on crystal collimation, de- were recorded for the first time in the LHC√ at a centre-of- scribed elsewhere [3]. This tight schedule was only feasible mass energy per colliding nucleon pair of sNN = 5.44 TeV. Physics data taking started 9.5hafter the first injection of by drastically reducing the complexity of the set-up follow- xenon beams and lasted a total of 6h. The integrated lumi- ing the model of the p–Pb pilot run in September 2012 [4,5] nosity delivered to the four LHC experiments was sufficient when first injection, validation and physics data-taking were that new physics results can be expected soon. We provide achieved within a single fill. -

XENON X-1100 High-Intensity Pulsed Light System
XENON X-1100 High-Intensity Pulsed Light System The low cost R&D tool for investigating the applicability of Pulsed Light for new and emerging applications. The tool that researchers, scientists and engineers have been looking for is here. An easy-to-use photonic system developed by XENON that will open new doors and lead to new discoveries. The power of Pulsed Light In virtually all disciplines of science and technology there are applications where precise delivery of energy is demanded. One less studied energy delivery mechanism is the use of high-intensity pulsed light. At present the most prevalent example of this is in the use of lasers. However, the point source, coherent and single wavelength nature of lasers, are often not suited to the majority of challenges facing many industries. In these situations, it is crucial to have a broad spectra source so as not to be restricted in choosing materials with specific absorption characteristics. Because XENON sources generate light which extends from the deep UV to IR and often with peak pulse powers measured in megawatts, the ability of these sources to perform tasks such as breaking chemical bonds, sintering, ablating or sterilizing is highly realizable. The high peak powers generated by XENON’s production level systems are possible by tightly controlling the pulse durations measured from a few microseconds to milliseconds. Until now there was no practical method of generating this intense pulsed light in a low-cost R&D tool that was safe, compact and versatile. XENON has developed a system to address this challenge based on over 50 years of exper- tise in the Pulsed Light industry. -

Pharmacology – Inhalant Anesthetics
Pharmacology- Inhalant Anesthetics Lyon Lee DVM PhD DACVA Introduction • Maintenance of general anesthesia is primarily carried out using inhalation anesthetics, although intravenous anesthetics may be used for short procedures. • Inhalation anesthetics provide quicker changes of anesthetic depth than injectable anesthetics, and reversal of central nervous depression is more readily achieved, explaining for its popularity in prolonged anesthesia (less risk of overdosing, less accumulation and quicker recovery) (see table 1) Table 1. Comparison of inhalant and injectable anesthetics Inhalant Technique Injectable Technique Expensive Equipment Cheap (needles, syringes) Patent Airway and high O2 Not necessarily Better control of anesthetic depth Once given, suffer the consequences Ease of elimination (ventilation) Only through metabolism & Excretion Pollution No • Commonly administered inhalant anesthetics include volatile liquids such as isoflurane, halothane, sevoflurane and desflurane, and inorganic gas, nitrous oxide (N2O). Except N2O, these volatile anesthetics are chemically ‘halogenated hydrocarbons’ and all are closely related. • Physical characteristics of volatile anesthetics govern their clinical effects and practicality associated with their use. Table 2. Physical characteristics of some volatile anesthetic agents. (MAC is for man) Name partition coefficient. boiling point MAC % blood /gas oil/gas (deg=C) Nitrous oxide 0.47 1.4 -89 105 Cyclopropane 0.55 11.5 -34 9.2 Halothane 2.4 220 50.2 0.75 Methoxyflurane 11.0 950 104.7 0.2 Enflurane 1.9 98 56.5 1.68 Isoflurane 1.4 97 48.5 1.15 Sevoflurane 0.6 53 58.5 2.5 Desflurane 0.42 18.7 25 5.72 Diethyl ether 12 65 34.6 1.92 Chloroform 8 400 61.2 0.77 Trichloroethylene 9 714 86.7 0.23 • The volatile anesthetics are administered as vapors after their evaporization in devices known as vaporizers. -

Unique Properties of Water!
Name: _______ANSWER KEY_______________ Class: _____ Date: _______________ Unique Properties of Water! Word Bank: Adhesion Evaporation Polar Surface tension Cohesion Freezing Positive Universal solvent Condensation Melting Sublimation Dissolve Negative 1. The electrons are not shared equally between the hydrogen and oxygen atoms of water creating a Polar molecule. 2. The polarity of water allows it to dissolve most substances. Because of this it is referred to as the universal solvent 3. Water molecules stick to other water molecules. This property is called cohesion. 4. Hydrogen bonds form between adjacent water molecules because the positive charged hydrogen end of one water molecule attracts the negative charged oxygen end of another water molecule. 5. Water molecules stick to other materials due to its polar nature. This property is called adhesion. 6. Hydrogen bonds hold water molecules closely together which causes water to have high surface tension. This is why water tends to clump together to form drops rather than spread out into a thin film. 7. Condensation is when water changes from a gas to a liquid. 8. Sublimation is when water changes from a solid directly to a gas. 9. Freezing is when water changes from a liquid to a solid. 10. Melting is when water changes from a solid to a liquid. 11. Evaporation is when water changes from a liquid to a gas. 12. Why does ice float? Water expands as it freezes, so it is LESS DENSE AS A SOLID. 13. What property refers to water molecules resembling magnets? How are these alike? Polar bonds create positive and negative ends of the molecule. -

Xenon Gas Xe Safety Data Sheet SDS P4677
Xenon Safety Data Sheet P-4677 This SDS conforms to U.S. Code of Federal Regulations 29 CFR 1910.1200, Hazard Communication. Date of issue: 01/01/1979 Revision date: 10/24/2016 Supersedes: 10/02/2014 SECTION: 1. Product and company identification 1.1. Product identifier Product form : Substance Name : Xenon CAS No : 7440-63-3 Formula : Xe Other means of identification : Xenon 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : Industrial use. Use as directed. 1.3. Details of the supplier of the safety data sheet Praxair, Inc. 10 Riverview Drive Danbury, CT 06810-6268 - USA T 1-800-772-9247 (1-800-PRAXAIR) - F 1-716-879-2146 www.praxair.com 1.4. Emergency telephone number Emergency number : Onsite Emergency: 1-800-645-4633 CHEMTREC, 24hr/day 7days/week — Within USA: 1-800-424-9300, Outside USA: 001-703-527-3887 (collect calls accepted, Contract 17729) SECTION 2: Hazard identification 2.1. Classification of the substance or mixture GHS-US classification Compressed gas H280 2.2. Label elements GHS-US labeling Hazard pictograms (GHS-US) : GHS04 Signal word (GHS-US) : WARNING Hazard statements (GHS-US) : H280 - CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED OSHA-H01 - MAY DISPLACE OXYGEN AND CAUSE RAPID SUFFOCATION Precautionary statements (GHS-US) : P202 - Do not handle until all safety precautions have been read and understood P271+P403 - Use and store only outdoors or in a well-ventilated place CGA-PG05 - Use a back flow preventive device in the piping CGA-PG10 - Use only with equipment rated for cylinder pressure CGA-PG06 - Close valve after each use and when empty CGA-PG02 - Protect from sunlight when ambient temperature exceeds 52°C (125°F) 2.3. -

Lung Ventilation - Xenon
Lung Ventilation - Xenon Special Instructions Patients with severe dyspnea may not be able to tolerate this examination. To be performed only at UNMH. Radiopharmaceutical: Xe-133 gas Dose (Adult/Pediatric): Refer to Nuclear Medicine Dose Chart Route of Administration: Inhalation from a Xe-133 gas delivery system. Patient Preparation: None. Equipment Setup: Gamma Camera: ZOOM as appropriate for small children Collimator: Orbiter: LEAP SPECT-CT/ECAM/Evo/Symbia E: High resolution Computer setup: • Static acquisition • 128 x 128 matrix • Zoom 1.0 (greater for small children)—ensure that the lungs fill a significant portion of the detector for small patients • 10 sec/image for inspiration • 30 sec/image for all other images Patient Positioning: Orbiter: • Seated upright on a stool (preferred) with the detector posterior to the patient. Supine if necessary All others: • Supine Procedure: • If the patient is able to sit upright on a stool for the examination, perform the ventilation portion of the examination on the Orbiter if available. • Before beginning the study, explain the breathing maneuvers required; the patient should rehearse these maneuvers. • Place the mask on the patient and ensure that it fits snugly. • Inspiration (Single-breath) Image: • Instruct the patient to exhale completely and then to take a deep breath as the Xe-133 gas flows in. • The patient should hold his/her breath for 10 sec (if possible) while posterior (and anterior if on a dual-headed camera) imaging is performed. Revised 2017-01 Lung Ventilation - Xenon (continued) • Equilibrium (Rebreathing/Wash-in) Images: • The patient should then breathe normally for 3 images (90 seconds). -

Periodic Table of the Elements Notes
Periodic Table of the Elements Notes Arrangement of the known elements based on atomic number and chemical and physical properties. Divided into three basic categories: Metals (left side of the table) Nonmetals (right side of the table) Metalloids (touching the zig zag line) Basic Organization by: Atomic structure Atomic number Chemical and Physical Properties Uses of the Periodic Table Useful in predicting: chemical behavior of the elements trends properties of the elements Atomic Structure Review: Atoms are made of protons, electrons, and neutrons. Elements are atoms of only one type. Elements are identified by the atomic number (# of protons in nucleus). Energy Levels Review: Electrons are arranged in a region around the nucleus called an electron cloud. Energy levels are located within the cloud. At least 1 energy level and as many as 7 energy levels exist in atoms Energy Levels & Valence Electrons Energy levels hold a specific amount of electrons: 1st level = up to 2 2nd level = up to 8 3rd level = up to 8 (first 18 elements only) The electrons in the outermost level are called valence electrons. Determine reactivity - how elements will react with others to form compounds Outermost level does not usually fill completely with electrons Using the Table to Identify Valence Electrons Elements are grouped into vertical columns because they have similar properties. These are called groups or families. Groups are numbered 1-18. Group numbers can help you determine the number of valence electrons: Group 1 has 1 valence electron. Group 2 has 2 valence electrons. Groups 3–12 are transition metals and have 1 or 2 valence electrons. -
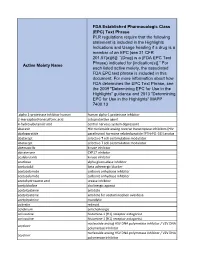
Active Moiety Name FDA Established Pharmacologic Class (EPC) Text
FDA Established Pharmacologic Class (EPC) Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: “(Drug) is a (FDA EPC Text Phrase) indicated for [indication(s)].” For Active Moiety Name each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13. .alpha. -

Pharmacokinetics and Pharmacology of Drugs Used in Children
Drug and Fluid Th erapy SECTION II Pharmacokinetics and Pharmacology of Drugs Used CHAPTER 6 in Children Charles J. Coté, Jerrold Lerman, Robert M. Ward, Ralph A. Lugo, and Nishan Goudsouzian Drug Distribution Propofol Protein Binding Ketamine Body Composition Etomidate Metabolism and Excretion Muscle Relaxants Hepatic Blood Flow Succinylcholine Renal Excretion Intermediate-Acting Nondepolarizing Relaxants Pharmacokinetic Principles and Calculations Atracurium First-Order Kinetics Cisatracurium Half-Life Vecuronium First-Order Single-Compartment Kinetics Rocuronium First-Order Multiple-Compartment Kinetics Clinical Implications When Using Short- and Zero-Order Kinetics Intermediate-Acting Relaxants Apparent Volume of Distribution Long-Acting Nondepolarizing Relaxants Repetitive Dosing and Drug Accumulation Pancuronium Steady State Antagonism of Muscle Relaxants Loading Dose General Principles Central Nervous System Effects Suggamadex The Drug Approval Process, the Package Insert, and Relaxants in Special Situations Drug Labeling Opioids Inhalation Anesthetic Agents Morphine Physicochemical Properties Meperidine Pharmacokinetics of Inhaled Anesthetics Hydromorphone Pharmacodynamics of Inhaled Anesthetics Oxycodone Clinical Effects Methadone Nitrous Oxide Fentanyl Environmental Impact Alfentanil Oxygen Sufentanil Intravenous Anesthetic Agents Remifentanil Barbiturates Butorphanol and Nalbuphine 89 A Practice of Anesthesia for Infants and Children Codeine Antiemetics Tramadol Metoclopramide Nonsteroidal Anti-infl ammatory Agents 5-Hydroxytryptamine -

Using Xenon-133 and a Scintillation Camera to Evaluate Pulmonary Function
USING XENON-133 AND A SCINTILLATION CAMERA TO EVALUATE PULMONARY FUNCTION Merle K. Loken and Hugh D. Westgate University of Minnesota Hospitals, Minneapolis, Minnesota During the past several years considerable atten monary-function laboratory (6—9). Thus because of tion has been given to evaluating pulmonary disease their universal availability, the noble gases are being using radioisotopic techniques. The majority of the used more extensively, and, of these gases, ‘33Xehas radioactive preparations used for these studies can the best physical characteristics (5, 10—13). be divided into two general categories : colloidal In this paper we report our preliminary experience aggregates of albumin and radioactive gases. Macro using an Anger scintillation camera to record pul aggregates labeled with 1311or 99@'Tchave been used monary uptake and clearance of ‘33Xein patients extensivelyfor determining regional pulmonary per referred to our service for pulmonary-function evalu fusion and, as such, are useful for evaluating patients ation. The radioactive xenon was administered either with suspected pulmonary emboli (1—5). The dis by inhalation or intravenous injection. tribution of these macroaggregates in the lung is usually determined by conventional rectilinear scan MATERIALS AND METHODS ning although the scintillation camera has also been We obtain 133Xe in 1-curie ampules at approxi used effectively (4,5). mately biweekly intervals from Oak Ridge National To obtain information on ventilation and diffusion Laboratory. The gas is contained in a volume of one of the radioactive gases—oxygen, carbon dioxide, about 5 cc at a pressure of about 10 mmHg (Fig. 1). krypton or xenon—must be used. -

Xenon-Tried-Tested-And-True.Pdf
Tried, tested and true: Why Xenon illumination is the preferred choice over laser phosphor for mainstream cinema Xenon – cinema’s workhorse With 45 years of use and 1.5 million installations worldwide, Christie® Xenolite® lamps are a constant in the cinema industry. Producing impressive light output, accurate color reproduction and boasting 99.999% reliability, this “lightning in a bottle” technology is the dependable workhorse trusted by exhibitors everywhere. Laser phosphor cinema projectors – not to be confused with RGB laser projectors – have been touted as a viable option to replace Xenon-based systems. This has resulted in cinema owners being faced with a decision. Should they continue using Xenon lamps, or make the switch to laser phosphor? Our answer to this is simple. For mainstream theatres, laser phosphor cinema projectors do not provide the performance advantages to justify them as an acceptable option over Xenon-based systems. Brightness over 30,000 hours 20K lumen-class, Xenon versus laser phosphor* 110% (Number of lamps used) (14 fL) 100% 1 2 3 4 5 6 7 8 9 10 11 Xenon 90% 11 fL typical (11 fL) 80% â Below DCI standards Just over 1 year á 70% of operation (based on 10 hours per day) 60% DCI Laser phosphor 7.0 fL typical ed brightness 50% ent of rc quir Pe re 0% 052,800 ,600 8,400 11,200 14,000 16,800 19,600 22,400 25,200 28,000 30,800 Hours of operation * ~20m wide screen * ~1.8 gain screen Laser phosphor – not suitable for cinema laser phosphor projectors. -

The Oxygen and Carbon Dioxide Compensation Points of C3
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 11230-11233, November 1995 Plant Biology The oxygen and carbon dioxide compensation points of C3 plants: Possible role in regulating atmospheric oxygen (photosynthetic carbon/02 inhibition/photorespiration/Nicotiana tobacum/Spinacea oleracea) N. E. TOLBERT*, C. BENKERt, AND E. BECKt *Department of Biochemistry, Michigan State University, East Lansing, MI 48824; and tLehrstuhl Fur Pflanzenphysiologie, Universitat Bayreuth, 95440 Bayreuth, Germany Contributed by N. E. Tolbert, August 15, 1995 ABSTRACT The 02 and CO2 compensation points (02 F bisphosphate to sustain the C2 cycle. Refixation of CO2 and C02 F) of plants in a closed system depend on the ratio of generates the same amount of 02 as taken up during the C2 CO2 and 02 concentrations in air and in the chloroplast and cycle. There is no net CO2 and 02 gas exchange during the specificities of ribulose bisphosphate carboxylase/oxyge- photorespiration (11) unless the complete C2 cycle is blocked nase (Rubisco). The photosynthetic 02 F is defined as the or metabolically interrupted by accumulation or removal of atmospheric 02 level, with a given CO2 level and temperature, products such as glycine or serine. Photorespiration dissipates at which net 02 exchange is zero. In experiments with C3 excess photosynthetic capacity (ATP and NADPH) without plants, the 02 F with 220 ppm CO2 is 23% 02; 02 F increases CO2 reduction or net 02 change. Photosynthetic carbon me- to 27% with 350 ppm CO2 and to 35% 02 with 700 ppm CO2. tabolism is a competition between CO2 and 02 for the dual At 02 levels below the 02 F, C02 uptake and reduction are activities of Rubisco, based on the ratio of CO2 and 02 accompanied by net 02 evolution.