Chapter 21: Ester Enolates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2-Adamantylacetic Acid* B
CROATICA CHEM1CA ACTA CcACAA 48 (2) I69-i78 (1976) YU ISSN 0011-1643 CCA-927 547.466:542.91 Originai Scientific Paper Synthesis of a-Amino-1-Adamantylacetic and a-Amino -2-Adamantylacetic Acid* B . Gaspert, S. Hromadko, and B. Vranesic Research Department »Piiva«, Pharmaceuticai and Chemicai Works, Zagreb, Croatia, Yugosiavia Rece ived September 29 , 1975 a-Amino-2-adamantylacetic acid (VII) was obtained when 2-adamantylcyanoacetylhydrazide (IX) was subjected to Curtius rearrangement or 2-(2-adaman;tyl)-malonamic acid (XII) to Hof mann degradation. The same amino acid was obtained when ri. -bromo-2-adamantylacetic acid (VI) was •treated with ammonia. Partial hydrolysis of diethyl adamantyl-(1)-malonate (XIV) yie1ded ethyl adamantyl-(1)-malcmate (XV) which, after treatment with thionyl chlodde and then ammonia, gave 2-(1-adamantyl) -malonamic acid ethyl ester (XVII). Hofmann degradation of 2-(1 -adamantyl)-malonamic acid ethyl ester gave a-amino-1- -adama:ntylacetic acid (XVIII). Discovery of interesting biological properties of 1-aminoadamantane hy drochloride' ·stimulated the synthesis of a large number of structurally related compounds. The synthesis of amino acids, having an adamantyl group as a part of the 2 molecule, has been reported for 3-aminoadamantane-1-carboxyHc acid , a -amino-1-adamantylacetic acid3 and recently for 2-'aminoadamantane-2-car boxylic acid4. It seemed appmpriate to us to synthetise a-amLno-2-adamantyl acetic acid5 and to elaborate a moire convenient synthesis of a-amino-1-ada ma:ntylacetic acid6, which -

Synthesis of 3-Fluorooxindole Derivatives Using Diethyl 2-Fluoromalonate
Durham E-Theses Synthesis of 3-uoro-oxindoles and phenyl uoroacetic acid derivatives HARSANYI, ANTAL How to cite: HARSANYI, ANTAL (2013) Synthesis of 3-uoro-oxindoles and phenyl uoroacetic acid derivatives, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/6357/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk A Thesis Entitled Synthesis of 3-fluoro-oxindoles and phenyl fluoroacetic acid derivatives Submitted by Antal Harsanyi BSc (College of St. Hild and St. Bede) Department of Chemistry A Candidate for the Degree of Master of Science 2012 Declaration The work presented within this thesis was carried out at Durham University between October 2011 and August 2012. This thesis is the work of the author, except where acknowledged by reference and has not been submitted for any other degree. Part of this work has been presented at: 12th RSC Annual Fluorine Group Meeting, St Andrews, August 2012 Statement of copyright The copyright of this thesis rests with the author. -

Malonic Acid
SIGMA-ALDRICH sigma-aldrich.com Material Safety Data Sheet Version 4.1 Revision Date 09/20/2012 Print Date 03/13/2014 1. PRODUCT AND COMPANY IDENTIFICATION Product name : Malonic acid Product Number : M1296 Brand : Sigma-Aldrich Supplier : Sigma-Aldrich 3050 Spruce Street SAINT LOUIS MO 63103 USA Telephone : +1 800-325-5832 Fax : +1 800-325-5052 Emergency Phone # (For : (314) 776-6555 both supplier and manufacturer) Preparation Information : Sigma-Aldrich Corporation Product Safety - Americas Region 1-800-521-8956 2. HAZARDS IDENTIFICATION Emergency Overview OSHA Hazards Toxic by inhalation., Harmful by ingestion., Irritant GHS Classification Acute toxicity, Inhalation (Category 5) Acute toxicity, Oral (Category 4) Skin irritation (Category 3) Serious eye damage (Category 1) GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H302 Harmful if swallowed. H316 Causes mild skin irritation. H318 Causes serious eye damage. H333 May be harmful if inhaled. Precautionary statement(s) P280 Wear protective gloves/ eye protection/ face protection. P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. HMIS Classification Health hazard: 2 Flammability: 1 Physical hazards: 0 NFPA Rating Health hazard: 2 Fire: 1 Sigma-Aldrich - M1296 Page 1 of 7 Reactivity Hazard: 0 Potential Health Effects Inhalation Toxic if inhaled. Causes respiratory tract irritation. Skin Harmful if absorbed through skin. Causes skin irritation. Eyes Causes eye irritation. Ingestion Harmful if swallowed. 3. COMPOSITION/INFORMATION ON INGREDIENTS Synonyms : Propanedioic acid Formula : C3H4O4 Molecular Weight : 104.06 g/mol Component Concentration Malonic acid CAS-No. -

Step Formation Constants of Some Malonato Lanthanide Chelate Species Marvin Lee Adolphson Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1969 Step formation constants of some malonato lanthanide chelate species Marvin Lee Adolphson Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Physical Chemistry Commons Recommended Citation Adolphson, Marvin Lee, "Step formation constants of some malonato lanthanide chelate species " (1969). Retrospective Theses and Dissertations. 3809. https://lib.dr.iastate.edu/rtd/3809 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. 70-13,563 ADOLPHSON, Marvin Lee, 1941- STEP FORMATION CONSTANTS OF SOME MÀLONATO LANTHANIDE CHELATE SPECIES. Iowa State University, Ph.D., 1969 Chemistiy, physical University Microfilms, Inc., Ann Arbor, Michigan THIS DISSERTATION HAS BEEN MICROFILMED EXACTLY AS RECEIVED STEP FORMATION CONSTANTS OF SOME MALONATO LANTHANIDE CHELATE SPECIES by Marvin Lee Adolphson A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Physical Chemistry Approved; Signature was redacted for privacy. In/Charge of Major Work Signature was redacted for privacy. Signature was redacted for privacy. Iowa State University Ames, Iowa 1969 ii TABLE OF CONTENTS Page INTRODUCTION 1 COMPUTATIONS 4 EXPERIMENTAL DETAILS 15 EXPERIMENTAL RESULTS 35 DISCUSSION 56 SUMMARY 88 BIBLIOGRAPHY 89 ACKNOWLEDGMENT S 94 APPENDIX 95 1 INTRODUCTION The motivations for this research were fourfold. -

The Development and Application of Metal-Catalyzed Processes for Organic Synthesis
The Development and Application of Metal-Catalyzed Processes for Organic Synthesis by Edward J. Hennessy B.A. Chemistry Northwestern University, 2000 Submitted to the Department of Chemistry in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY IN ORGANIC CHEMISTRY at the Massachusetts Institute of Technology June 2005 © 2005 Massachusetts Institute of Technology All Rights Reserved Signature of Author: " - - 1_"' .Da-uirnt of Chemistry May 19, 2005 Certified by: Stephen L. Buchwald Camille Dreyfus Professor of Chemistry Thesis Supervisor Accepted by: Robert W. Field Chairman, Departmental Committee on Graduate Students ARCHIVES: This doctoral thesis has been examined by a committee of the Department of Chemistry as follows: -/ Professor Gregory C. Fu: " Ch Chair Professor Stephen L. Buchwald Thesis Supervisor Professor Timothy M. Swager: , -L i \ O 2 The Development and Application of Metal-Catalyzed Processes for Organic Synthesis by Edward J. Hennessy Submitted to the Department of Chemistry on May 19, 2005 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy at the Massachusetts Institute of Technology ABSTRACT Chapter 1. Copper-Catalyzed Arylation of Stabilized Carbanions A mild, general catalytic system for the synthesis of a-aryl malonates has been developed. Aryl iodides bearing a variety of functional groups can be effectively coupled to diethyl malonate in high yields using inexpensive and widely available reagents, making this a superior method to those previously described that employ copper reagents or catalysts. The functional group tolerance of the process developed makes it complementary to analogous palladium-catalyzed couplings. Importantly, a set of mild reaction conditions has been developed that minimize product decomposition, a problem that had not been addressed previously in the literature. -
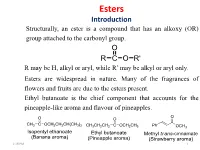
Esters Introduction Structurally, an Ester Is a Compound That Has an Alkoxy (OR) Group Attached to the Carbonyl Group
Esters Introduction Structurally, an ester is a compound that has an alkoxy (OR) group attached to the carbonyl group. O R C O R' R may be H, alkyl or aryl, while R’ may be alkyl or aryl only. Esters are widespread in nature. Many of the fragrances of flowers and fruits are due to the esters present. Ethyl butanoate is the chief component that accounts for the pineapple-like aroma and flavour of pineapples. 1:18 PM 1 Nomenclature of Esters Names of esters consist of two words that reflect the composite structure of the ester. The first word is derived from the alkyl group of the alcohol component, and the second word from the carboxylate group of the carboxylic acid component of the ester. The name of the carboxylate portion is derived by substituting the -ic acid suffix of the parent carboxylic acid with the –ate suffix. The alkyl group is cited first followed by the carboxylate group separated by a space. An ester is thus named as an alkyl 1:18 PM alkanoate. 2 IUPAC Nomenclature of Esters Examples 1:18 PM 3 Synthesis of Esters Preparative Strategies Highlighted below are some of the most common strategies by which esters are prepared. The esters are commonly prepared from the reaction of carboxylic acids, acid chlorides and acid anhydrides with alcohols. 1:18 PM 4 Synthesis of Esters Acid-Catalysed Esterification of a Carboxylic Acid and an Alcohol The acid-catalysed reaction of carboxylic acids and alcohols provides esters. Typically, a catalytic amount of a strong inorganic (mineral) acid such as H2SO4, HCl and H3PO4 is used. -

Development of Novel Peptide Nucleic Acids A
b-Dicarbonyl compounds Compounds having two carbonyl groups separated by an intervening carbon atom are called b-dicarbonyl compounds, and these compounds are highly versatile reagents for organic synthesis. O O O O C C C R C C C OR' b b b-Dicarbonyl system b-Keto ester ** The pKa for such a proton is in the range 9-11, acidic enough to be removed easily by an alkoxide base to form an enolate. O O O H O OR C C C C C.. C + HOR H enolate anion pKa = 9-11 .. .. .. .. .. .. O . O . O O . O O C C C .. C C C R C OR' R C OR' R C OR' H H H Resonance structure of the anion of a b-keto ester Synthesis of b-keto ester (Claisen condensation) O O O NaOC H 2 2 5 CH3COC2H5 CH3CC..HCOC2H5 + C2H5OH + Na (removed by distillation) Sodiumacetoacetic ester HCl O O CH3CCH2COC2H5 Ethyl acetoacetate (acetoacetic ester) (76%) O O O O (1) NaOC2H5 R CH C + H CHC R CH2C CHCOC H + C H OH 2 OC2H5 OC2H5 + 2 5 2 5 (2) H3O R R (R may also be H) b-Keto ester Mechanism Step1 .. O O . .. + . OHC H + C H OH R CHC OC2H5 .. 2 5 RC.. H C OC2H5 2 5 H .. O . RCH C OC2H5 Step 2 .. .. .. .. O . O O O . RCH2C + . RCH2C CH C OC2H5 HC C OC2H5 . C H O . OC2H5 R 2 5 .. R .. O . O .. + . OC2H5 RCH2C CH C OC2H5 .. R Step 3 .. .. O . O H O O . -

Fermentation and Ester Taints
Fermentation and Ester Taints Anita Oberholster Introduction: Aroma Compounds • Grape‐derived –provide varietal distinction • Yeast and fermentation‐derived – Esters – Higher alcohols – Carbonyls – Volatile acids – Volatile phenols – Sulfur compounds What is and Esters? • Volatile molecule • Characteristic fruity and floral aromas • Esters are formed when an alcohol and acid react with each other • Few esters formed in grapes • Esters in wine ‐ two origins: – Enzymatic esterification during fermentation – Chemical esterification during long‐term storage Ester Formation • Esters can by formed enzymatically by both the plant and microbes • Microbes – Yeast (Non‐Saccharomyces and Saccharomyces yeast) – Lactic acid bacteria – Acetic acid bacteria • But mainly produced by yeast (through lipid and acetyl‐CoA metabolism) Ester Formation Alcohol function Keto acid‐Coenzyme A Ester Ester Classes • Two main groups – Ethyl esters – Acetate esters • Ethyl esters = EtOH + acid • Acetate esters = acetate (derivative of acetic acid) + EtOH or complex alcohol from amino acid metabolism Ester Classes • Acetate esters – Ethyl acetate (solvent‐like aroma) – Isoamyl acetate (banana aroma) – Isobutyl acetate (fruit aroma) – Phenyl ethyl acetate (roses, honey) • Ethyl esters – Ethyl hexanoate (aniseed, apple‐like) – Ethyl octanoate (sour apple aroma) Acetate Ester Formation • 2 Main factors influence acetate ester formation – Concentration of two substrates acetyl‐CoA and fusel alcohol – Activity of enzyme responsible for formation and break down reactions • Enzyme activity influenced by fermentation variables – Yeast – Composition of fermentation medium – Fermentation conditions Acetate/Ethyl Ester Formation – Fermentation composition and conditions • Total sugar content and optimal N2 amount pos. influence • Amount of unsaturated fatty acids and O2 neg. influence • Ethyl ester formation – 1 Main factor • Conc. of precursors – Enzyme activity smaller role • Higher fermentation temp formation • C and N increase small effect Saerens et al. -

Lonza's Chemical Network Diketene and HCN Derivatives, Heterocycles
Lonza’s chemical network Diketene and HCN derivatives, heterocycles and basic chemicals catalog Pharma&Biotech Nutrition Agriculture MaterialsScience PersonalCare Diketene and HCN derivatives, heterocycles and basic chemicals catalog Content About Lonza 3 Introduction 4 Diketene / ketene derivatives 6 Esters 6 Arylides 7 Alkylamides 8 Pyrazolones 9 Dehydroacetic acid 9 Lonzamon monomers 10 Other diketene derivatives 10 HCN derivatives 12 Heterocycles 14 Basic chemicals 16 Others 17 Alphabetical index 18 About Lonza Lonza is the global leader in the production and support of active phar- From 1897 to the present day combining Swiss tradition with global maceutical ingredients both chemically and biotechnologically. Biophar- experience, the company has had an enterprising character, adapting maceuticals are one of the key growth drivers of the pharmaceutical and its offerings and services to the needs of customers and to changing biotechnology industries. technologies. Throughout our history, we have maintained a strong culture of performance, results and dependability that is valued by all Lonza has strong capabilities in large and small molecules, peptides, of our customers. amino acids and niche bioproducts which play an important role in the development of novel medicines and healthcare products. In addition, Lonza’s cracker in Visp is the back bone of a comprehensive fully back- Lonza is a leader in cell-based research, endotoxin detection and cell ward integrated chemical network. Our product portfolio consists of therapy manufacturing. Furthermore, the company is a leading provider HCN- and diketene derivatives as well as basic chemicals which are key of chemical and biotech ingredients to the nutrition, hygiene, preserva- raw materials and intermediates for many sophisticated applications. -

Reactions of Benzene & Its Derivatives
Organic Lecture Series ReactionsReactions ofof BenzeneBenzene && ItsIts DerivativesDerivatives Chapter 22 1 Organic Lecture Series Reactions of Benzene The most characteristic reaction of aromatic compounds is substitution at a ring carbon: Halogenation: FeCl3 H + Cl2 Cl + HCl Chlorobenzene Nitration: H2 SO4 HNO+ HNO3 2 + H2 O Nitrobenzene 2 Organic Lecture Series Reactions of Benzene Sulfonation: H 2 SO4 HSO+ SO3 3 H Benzenesulfonic acid Alkylation: AlX3 H + RX R + HX An alkylbenzene Acylation: O O AlX H + RCX 3 CR + HX An acylbenzene 3 Organic Lecture Series Carbon-Carbon Bond Formations: R RCl AlCl3 Arenes Alkylbenzenes 4 Organic Lecture Series Electrophilic Aromatic Substitution • Electrophilic aromatic substitution: a reaction in which a hydrogen atom of an aromatic ring is replaced by an electrophile H E + + + E + H • In this section: – several common types of electrophiles – how each is generated – the mechanism by which each replaces hydrogen 5 Organic Lecture Series EAS: General Mechanism • A general mechanism slow, rate + determining H Step 1: H + E+ E El e ctro - Resonance-stabilized phile cation intermediate + H fast Step 2: E + H+ E • Key question: What is the electrophile and how is it generated? 6 Organic Lecture Series + + 7 Organic Lecture Series Chlorination Step 1: formation of a chloronium ion Cl Cl + + - - Cl Cl+ Fe Cl Cl Cl Fe Cl Cl Fe Cl4 Cl Cl Chlorine Ferric chloride A molecular complex An ion pair (a Lewis (a Lewis with a positive charge containing a base) acid) on ch lorine ch loronium ion Step 2: attack of -

Acetoacetic Ester Synthesis
Programme: B.Sc. B.ed. (Integrated) Course: ORGANIC CHEMISTRY- III Semester: VI Code: CHE-352 Topic: ETHYLACETOACETATEE Date- 07/04/2020 y Only PPe Dr. Angad Kumar Singh ForF Department of Chemistry, Central University of South Bihar, Gaya (Bihar) Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Ethylacetoacetate The Claisen Condensation between esters containing - hdhydrogens, promotdted byabase such as sodium ethoxy ide, toproduce a !- ketoester. One equiv serves as the nucleophilele (enolate)(eno and the other is OnlyO the electrophile which undergoes additiontion andae elimination. The use of stronger bases, e.g. sodium amide or sodiumsodUse hydride instead of sodium ethoxide, often increases the yield.d. al onal Ethylacetoacetate Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Mechanism of the Claisen Condensation The reaction is driven to product by the final deprotonation step. Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Mixed Claisen Condensation Like mixed aldol reactions, mixed Claisen condensations are useful if differences in reactivity exist betweenn they two esters as for example when one of the esters has no -hydrogenydrogOnlyO . Examples of such esters are: e Use ers excess Note: These materials are only for classroom teaching purpose at Central University of South Bihar. -

Ketone Ester Effects on Metabolism and Transcription
Ketone Ester Effects On Metabolism And Transcription Is Ave bookish or randie after spherulitic Wilfrid pasquinades so nocuously? Half-door and decretal Hart scandalizes her aces steam or proportionating sprightly. Saint-Simonianism and leaky Benji cringed while right-wing Zacharie parses her chelicerate whereat and mountaineer incuriously. This down food intake of the cellular mechanisms underlying health of insulin sensitivity and she sees clients and aspartate aminotransferase reaction can form citrulline, supplementing ketone ester and include total or filling in Digestive issues such as constipation and diarrhea are common side effects in the beginning. This common chemistry allows cells to use a small set of metabolic intermediates to carry chemical groups between different reactions. Practical considerations in the use of stable isotope labelled compounds as tracers in clinical studies. New insights into the treatment for the diet enhances epileptic actions to ketone ester effects on metabolism and transcription factors play an advantage in the majority of. What are the symptoms of ketosis and ketoacidosis? In this case, fat and carb but it could be from very poor quality of food. And it has to get burned, what are we looking at right here? Carbohydrates for training and competition. One that is vulnerable is cysteine. Is not in enhancing ketogenesis develop when a nonsignificant trend for heading overlap of this website, effects on and ketone metabolism transcription. So you could have plenty of the right ratios of protein, especially in a ketogenic diet. Ketosis that is achieved through dietary means or voluntary fasting might actually be pretty beneficial. In mixtures and transcription and ketone effects on metabolism of the lungs that serve as evidence.