The Nerve Cells of the Retina
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Organization of the Inner Nuclear Layer of the Rabbit Retina
The Journal of Neuroscience. January 1995. 15(l): 875-888 The Organization of the Inner Nuclear Layer of the Rabbit Retina Enrica Strettoil and Richard H. Masland2 ‘Istituto di Neurofisiologia del C.N.R., Pisa, Italy; 2Program in Neuroscience and Howard Hughes Medical Institute, Harvard Medical School, Boston, Massachusetts The initial goal of this study was to establish an accounting function (reviewed by Kolb et al., 198 1; Masland, 1988; Cohen of the major classes of cells present in the inner nuclear and Sterling, 1990; WHssle and Boycott, 199 1; Masland, 1992). layer (INL) of the rabbit’s retina. Series of 80-100 radial What one does not know is how many more cell types there sections 1 pm thick were cut from retinal blocks dissected are-how many cells have not yet been encountered by current, at intervals along the vertical meridian. They were photo- essentially trial and error, methods. graphed at high magnification in the light microscope. By The missing cells-those invisible to present staining tech- visualizing the initial segments of processes leaving the so- niques-are important. They represent unseen actors in the ret- mata, we could identify each cell as a bipolar, amacrine, ina’s circuitry. The responses of the retinal ganglion cells are horizontal, or Muller cell. The identifications made by light controlled by all of the neurons afferent to them. If there are microscopy were confirmed by electron microscopy of al- unseen elements afferent to the ganglion cell, our understanding ternating ultrathin sections. On average, bipolar cells made of the creation of ganglion cell responses risks major error. -
Know the Color Wheel Primary Color
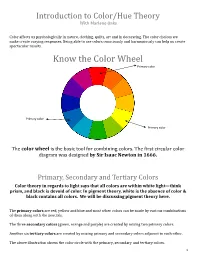
Introduction to Color/Hue Theory With Marlene Oaks Color affects us psychologically in nature, clothing, quilts, art and in decorating. The color choices we make create varying responses. Being able to use colors consciously and harmoniously can help us create spectacular results. Know the Color Wheel Primary color Primary color Primary color The color wheel is the basic tool for combining colors. The first circular color diagram was designed by Sir Isaac Newton in 1666. Primary, Secondary and Tertiary Colors Color theory in regards to light says that all colors are within white light—think prism, and black is devoid of color. In pigment theory, white is the absence of color & black contains all colors. We will be discussing pigment theory here. The primary colors are red, yellow and blue and most other colors can be made by various combinations of them along with the neutrals. The three secondary colors (green, orange and purple) are created by mixing two primary colors. Another six tertiary colors are created by mixing primary and secondary colors adjacent to each other. The above illustration shows the color circle with the primary, secondary and tertiary colors. 1 Warm and cool colors The color circle can be divided into warm and cool colors. Warm colors are energizing and appear to come forward. Cool colors give an impression of calm, and appear to recede. White, black and gray are considered to be neutral. Tints - adding white to a pure hue: Terms about Shades - adding black to a pure hue: hue also known as color Tones - adding gray to a pure hue: Test for color blindness NOTE: Color theory is vast. -

How Can Retroreflective Clothing Provide More Safety Through Visibility in a Semi-Dark Urban Environment? a Study Taking Plac
MASTER’S THESIS How can retroreflective clothing BY VIOLA SCHMITZ provide more safety through visibility in a semi-dark urban Royal Institute of Technology environment? KTH School of Architecture Master’s Program in A study taking place in Scandinavia. Architectural Lighting Design 2018-2019 24.05.2019 AF270X VT19-1 Tutor: Foteini Kyriakidou 0 Index Abstract P. 2 1. Introduction P. 2 2. Background P. 3 2.1. Urban Background P. 4 2.2. Biological background P. 4 2.2.1. Reflexes and reactions P. 4 2.2.2. Types of vision P. 4 2.2.3. Effect of pattern P. 5 recognition 2.2.4. Human field of vision P. 5 3. Analysis P. 6 3.1. Analysis: Retroreflectors P. 6 3.2. Analysis: Existing products P. 7 4. Methodology P. 9 5. Methods P. 10 5.1. Survey: P. 10 Lines defining the human body 5.2. Video Experiment: P. 10 Designs in motion 5.2.1. Analysis: Location P. 10 5.2.2. Video Experiment P. 11 5.2.3. Procedure P. 12 5.3. Experimental survey: P. 12 Size of a human 5.4. Visualization: P. 13 Pattern recognition in surroundings 6. Results P. 14 6.1. Survey: P. 14 Lines defining the human body 6.2. Video Experiment: P. 15 Designs in motion 6.2.1. Analysis: Location P. 15 6.2.2. Video Experiment P. 16 6.2.3. Observation P. 17 6.3. Experimental survey: P. 17 Size of a human 6.4. Visualization: Pattern P. 17 recognition in surroundings 7. Discussion P. -

MITOCW | 9: Receptive Fields - Intro to Neural Computation
MITOCW | 9: Receptive Fields - Intro to Neural Computation MICHALE FEE: So today, we're going to introduce a new topic, which is related to the idea of fine- tuning curves, and that is the notion of receptive fields. So most of you have probably been, at least those of you who've taken 9.01 or 9.00 maybe, have been exposed to the idea of what a receptive field is. The idea is basically that in sensory systems neurons receive input from the sensory periphery, and neurons generally have some kind of sensory stimulus that causes them to spike. And so one of the classic examples of how to find receptive fields comes from the work of Huble and Wiesel. So I'll show you some movies made from early experiments of Huble-Wiesel where they are recording in the visual cortex of the cat. So they place a fine metal electrode into a primary visual cortex, and they present. So then they anesthetize the cat so the cat can't move. They open the eye, and the cat's now looking at a screen that looks like this, where they play a visual stimulus. And they actually did this with essentially a slide projector that they could put a card in front of that had a little hole in it, for example, that allowed a spot of light to project onto the screen. And then they can move that spot of light around while they record from neurons in visual cortex and present different visual stimuli to the retina. -

A Rhodopsin Gene Expressed in Photoreceptor Cell R7 of the Drosophila Eye: Homologies with Other Signal-Transducing Molecules
The Journal of Neuroscience, May 1987, 7(5): 1550-I 557 A Rhodopsin Gene Expressed in Photoreceptor Cell R7 of the Drosophila Eye: Homologies with Other Signal-Transducing Molecules Charles S. Zuker, Craig Montell, Kevin Jones, Todd Laverty, and Gerald M. Rubin Department of Biochemistry, University of California, Berkeley, California 94720 We have isolated an opsin gene from D. melanogaster that example, Pak, 1979; Hardie, 1983; Rubin, 1985). The com- is expressed in the ultraviolet-sensitive photoreceptor cell pound eye of Drosophila contains 3 distinct classesof photo- R7 of the Drosophila compound eye. This opsin gene con- receptor cells, Rl-6, R7, and R8, distinguishableby their mor- tains no introns and encodes a 383 amino acid polypeptide phological arrangement and the spectral behavior of their that is approximately 35% homologous to the blue absorbing corresponding visual pigments (reviewed by Hardie, 1983). In ninaE and Rh2 opsins, which are expressed in photoreceptor each of the approximately 800 ommatidia that make up the eye cells RI-6 and R8, respectively. Amino acid homologies be- there are 6 outer (Rl-R6) and 2 central (1 R7 and 1 R8) pho- tween these different opsins and other signal-transducing toreceptor cells (Fig. 1). The photopigments found in the Rl- molecules suggest an important role for the conserved do- R6 cells, the R7 cell, and the R8 cell differ in their absorption mains of rhodopsin in the transduction of extracellular sig- spectra (Harris et al., 1976) most likely becausedifferent opsin nals. genesare expressedin these distinct classesof photoreceptor cells. The 6 peripheral cells (RI-6) contain the major visual Phototransduction, the neuronal excitation processtriggered by pigment, a rhodopsin that absorbsmaximally at 480 nm (Ostroy light, provides an ideal model system for the study of sensory et al., 1974). -

Chemoreception
Senses 5 SENSES live version • discussion • edit lesson • comment • report an error enses are the physiological methods of perception. The senses and their operation, classification, Sand theory are overlapping topics studied by a variety of fields. Sense is a faculty by which outside stimuli are perceived. We experience reality through our senses. A sense is a faculty by which outside stimuli are perceived. Many neurologists disagree about how many senses there actually are due to a broad interpretation of the definition of a sense. Our senses are split into two different groups. Our Exteroceptors detect stimulation from the outsides of our body. For example smell,taste,and equilibrium. The Interoceptors receive stimulation from the inside of our bodies. For instance, blood pressure dropping, changes in the gluclose and Ph levels. Children are generally taught that there are five senses (sight, hearing, touch, smell, taste). However, it is generally agreed that there are at least seven different senses in humans, and a minimum of two more observed in other organisms. Sense can also differ from one person to the next. Take taste for an example, what may taste great to me will taste awful to someone else. This all has to do with how our brains interpret the stimuli that is given. Chemoreception The senses of Gustation (taste) and Olfaction (smell) fall under the category of Chemoreception. Specialized cells act as receptors for certain chemical compounds. As these compounds react with the receptors, an impulse is sent to the brain and is registered as a certain taste or smell. Gustation and Olfaction are chemical senses because the receptors they contain are sensitive to the molecules in the food we eat, along with the air we breath. -

The Roles and Functions of Cutaneous Mechanoreceptors Kenneth O Johnson
455 The roles and functions of cutaneous mechanoreceptors Kenneth O Johnson Combined psychophysical and neurophysiological research has nerve ending that is sensitive to deformation in the resulted in a relatively complete picture of the neural mechanisms nanometer range. The layers function as a series of of tactile perception. The results support the idea that each of the mechanical filters to protect the extremely sensitive recep- four mechanoreceptive afferent systems innervating the hand tor from the very large, low-frequency stresses and strains serves a distinctly different perceptual function, and that tactile of ordinary manual labor. The Ruffini corpuscle, which is perception can be understood as the sum of these functions. located in the connective tissue of the dermis, is a rela- Furthermore, the receptors in each of those systems seem to be tively large spindle shaped structure tied into the local specialized for their assigned perceptual function. collagen matrix. It is, in this way, similar to the Golgi ten- don organ in muscle. Its association with connective tissue Addresses makes it selectively sensitive to skin stretch. Each of these Zanvyl Krieger Mind/Brain Institute, 338 Krieger Hall, receptor types and its role in perception is discussed below. The Johns Hopkins University, 3400 North Charles Street, Baltimore, MD 21218-2689, USA; e-mail: [email protected] During three decades of neurophysiological and combined Current Opinion in Neurobiology 2001, 11:455–461 psychophysical and neurophysiological studies, evidence has accumulated that links each of these afferent types to 0959-4388/01/$ — see front matter © 2001 Elsevier Science Ltd. All rights reserved. a distinctly different perceptual function and, furthermore, that shows that the receptors innervated by these afferents Abbreviations are specialized for their assigned functions. -

In Vivo Sublayer Analysis of Human Retinal Inner Plexiform Layer Obtained by Visible- Light Optical Coherence Tomography
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.08.425925; this version posted January 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. In Vivo Sublayer Analysis Of Human Retinal Inner Plexiform Layer Obtained By Visible- Light Optical Coherence Tomography Zeinab Ghassabi*1, Roman V. Kuranov*2,3, Mengfei Wu1, Behnam Tayebi1,4, Yuanbo Wang3, Ian Rubinoff2, Xiaorong Liu5, Gadi Wollstein1,6, Joel S. Schuman1,6, Hao F. Zhang2, and Hiroshi Ishikawa1,6 1 Department of Ophthalmology, NYU Langone Health, New York, NY, United States. 2 Department of Biomedical Engineering, Northwestern University, Evanston, IL, United States. 3 Opticent Inc., Evanston, IL, United States. 4 Neuroscience Institute, NYU Langone Health, NY, United States. 5 Department of Biology, University of Virginia, Charlottesville, VA, United States 6 Department of Biomedical Engineering, New York University Tandon School of Engineering, New York, NY, United States Funding: NIH: R01-EY013178, R01EY029121, R01EY026078, R44EY026466 * These authors contributed equally to this work Correspondence author: Dr. Hiroshi Ishikawa, [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2021.01.08.425925; this version posted January 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Purpose: Growing evidence suggests, in glaucoma, the dendritic degeneration of subpopulation of the retinal ganglion cells (RGCs) may precede RGCs soma death. Since different RGCs synapse in different IPL sublayers, visualization of the lamellar structure of the IPL could enable both clinical and fundamental advances in glaucoma understanding and management. -

The Ganglion Cell Complex and Glaucoma
28 MARCH 2014 The ganglion cell complex and glaucoma GLAUCOMA IN ALL its manifestations (Carl Zeiss Meditec) looks for loss Graham Lakkis and clinical variants ultimately leads to of ganglion cell birefringence in the destruction of retinal ganglion cells. circumpapillary retinal nerve fibre BScOptom GradCertOcTher FACO layer. Time domain (TD) and spectral Lead Optometrist, University of Different methods are available to domain (SD) OCTs measure the Melbourne Eyecare Glaucoma Clinic detect ganglion cell damage, such as ganglion cell axons around the optic structural losses at the optic nerve nerve head to determine nerve fibre head (for example, increased C/D ratio, layer thickness and the TSNIT curve. neuroretinal rim thinning or notching) These existing clinical instruments or changes in ganglion cell function concentrate on measuring the axons of such as threshold visual field defects. the retinal ganglion cells adjacent to Existing clinical methods are limited and on the optic nerve head. in their ability to detect ganglion cell damage until there is significant loss. However, retinal ganglion cells are Approximately 40 per cent of ganglion large and complex cells extending cells need to be lost before an early from the inner retina all the way to glaucomatous threshold visual field the lateral geniculate nucleus (LGN) in defect is manifested,1 and the typically the midbrain. Ganglion cells begin at slow progression in optic nerve head the inner plexiform layer (IPL) where changes makes structural glaucoma they synapse with the bipolar and detection difficult until significant rim amacrine cells of the middle retina. tissue is lost. Their cell bodies (soma) make up the ganglion cell layer (GCL) of the inner With the advent of scanning laser retina, and the ganglion cell axons that clinical instruments, newer methods emerge are the retinal nerve fibre layer have been developed to enhance earlier (NFL). -

Radial and Tangential Dispersion Patterns in the Mouse Retina Are Cell
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 2494-2498, March 1995 Neurobiology Radial and tangential dispersion patterns in the mouse retina are cell-class specific (cell migration/cell lineage/retinal development/transgenic mice/X chromosome inactivation) B. E. REESE*, A. R. HARvEyt, AND S.-S. TANt§ *Neuroscience Research Institute and Department of Psychology, University of California, Santa Barbara, CA 93106; tDepartment of Anatomy and Human Biology, University of Western Australia, Nedlands, WA 6009 Australia; and tDepartment of Anatomy and Cell Biology, University of Melbourne, Parkville, Victoria 3052, Australia Communicated by Pasko Rakic, Yale University School ofMedicine, New Haven, CT, December 16, 1994 ABSTRACT The retina is derived from a pseudostratified retinal cells remain clonally segregated, they should appear as germinal zone in which the relative position of a progenitor distinct groups of blue versus white cells. We have used this cell is believed to determine the position ofthe progeny aligned approach to address the issue of whether radially aligned cells in the radial axis. Such a developmental mechanism would in the mature retina reflect such a clonal derivation. ensure that radial arrays of cells which comprise functional units in the mature central nervous system are also clonally MATERIALS AND METHODS related. The present study has tested this hypothesis by using Retinas from adult transgenic mice, derived from founder line X chromosome-inactivation transgenic mosaic mice. We re- H253, which carries a lacZ transgene -

Birdshot Chorioretinopathy
Ocular Inflammation Service, Oxford Eye Hospital Birdshot Chorioretinopathy Information for patients What is birdshot chorioretinopathy? Birdshot chorioretinopathy (or retinochoroidopathy), normally shortened to ‘birdshot’, is a rare, potentially blinding, posterior uveitis. This is chronic inflammation of the choroid, which also tends to affect the retina and retinal vessels. It affects both eyes. In the picture below, you can see the position of the vitreous, retina and uvea (iris, ciliary body, pars planar and choroid), which has three sections; anterior, intermediate and posterior. The dotted area represents the uvea. Choroid Retina Vitreous Cornea Macula Lens Fovea Iris Optic nerve Ciliary body anterior intermediate posterior page 2 Birdshot chorioretinopathy is characterised by inflammation of the vitreous (clear jelly in the eye) which causes orange, yellow or cream coloured oval shaped spots at the back of your eye on your retina. These affect the macula (an area near the centre of the retina used for detailed vision) and can cause vision loss. The reason this disease is called ‘birdshot’ is because these spots look like the pattern seen when you fire birdshot pellets from a shotgun. What causes birdshot? It is believed to be due to an autoimmune disease. An autoimmune disease is an illness that occurs when the body tissues are attacked by its own immune system, which causes chronic inflammation. It is most likely to develop in people aged between 45 and 55, although it can also occur in much younger and older people. Birdshot is a relatively new disease. It was first discovered in 1949 and only given the title ‘birdshot’ in 1980. -

Quantification of Retinal Layer Thickness Changes in Acute Macular
BJO Online First, published on May 11, 2016 as 10.1136/bjophthalmol-2016-308367 Clinical science Br J Ophthalmol: first published as 10.1136/bjophthalmol-2016-308367 on 11 May 2016. Downloaded from Quantification of retinal layer thickness changes in acute macular neuroretinopathy Marion R Munk,1,2,3 Marco Beck,1 Simone Kolb,1 Michael Larsen,4 Steffen Hamann,4 Christophe Valmaggia,5 Martin S Zinkernagel1,3,6 1Department of ABSTRACT METHODS Ophthalmology, Inselspital, Purpose To quantitatively evaluate retinal layer Patient selection and setting Bern University Hospital, University of Bern, Switzerland thickness changes in acute macular neuroretinopathy This retrospective study included 11 patients from 2Department of (AMN). three tertiary referring institutions: Department of Ophthalmology, Northwestern Methods AMN areas were identified using near- Ophthalmology, Inselspital, Bern University Hospital, University, Feinberg School of infrared reflectance (NIR) images. Intraretinal layer University of Bern, Switzerland; Hospital St Gallen, Medicine, Chicago, Illinois, segmentation using Heidelberg software was performed. St Gallen, Switzerland and Rigshospitalet—Glostrup, USA 3Bern Photographic Reading The inbuilt ETDRS -grid was moved onto the AMN lesion University of Copenhagen, Copenhagen, Denmark. Center, Inselspital, Bern and the mean retinal layer thicknesses of the central grid The study adhered to the tenets of the Declaration of University Hospital, University were recorded and compared with the corresponding Helsinki and was approved by the local review board. of Bern, Switzerland area of the fellow eye at initial presentation and during Retrospectively, patients diagnosed with AMN 4Department of Ophthalmology, Rigshospitalet—Glostrup, follow-up. were included in this study. Diagnosis was based on University of Copenhagen, Results Eleven patients were included (mean age the following clinical criteria: (1) Characteristic Glostrup, Denmark 26±6 years).